| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
REACTION OF CO2 WITH CLEAN COAL TECHNOLOGY
ASH TO REDUCE TRACE ELEMENT MOBILITY
T.A. Tawfic, K.J. Reddy,
S.P. Gloss and J.I. Drever
Journal Article 1995 WWRC-95-13
In Water, Air and Soil Pollution
84:385-398, 1995
T.A. TAWFIC, K.J. REDDY and S.P. GLOSS
Wyoming Water Resources Center, PO. Box 3067, University of Wyoming, Laramie, WY 82071
U.S.A.
and
J.I. DREVER
Geology and Geophysics, P.O. Box 3006, University of Wyoming, Laramie, WY 82071,
U.S.A.
(Received 25 April, 1994; accepted in final form 28 November, 1994)
Abstract. The combustion of coal in power plants generates solids (e.g., fly ash, bottom ash) and flue gas (e.g., SOX, C02). New Clean Air Act mandated reduction of SOX emissions from coal burning power plants. As a result, a variety of Clean Coal Technologies (CCT) are implemented to comply with these amendments. However, most of the CCT processes transfer environmentally sensitive elements (e.g., As, Cd, Pb, Se) from flue gas to CCT ash. The objective of this study was to determine the effect of a pressurized C02 treatment on the chemistry of CCT ash. Three CCT ash samples, produced from lime injection, atmospheric fluidized bed combustion, and sodium carbonate injection processes were reacted under different C02 pressure treatment conditions. Treated and untreated samples were subjected to various experiments including, X-ray diffraction (XRD) analysis, calcium carbonate solubility studies, and trace element extraction studies. Factors influencing the efficiency of a C02 treatment for CCT ash samples include combustion process, moisture, C02 concentration, and pressure. The C02 pressure treatment resulted in the precipitation of calcite in CCT ash samples, and thus lowered the pH and the concentration of extractable trace elements (e.g., Cd, Pb, Cr, As, Se). Furthermore, we found that C02 pressure treatment was more effective for lime injection and atmospheric fluidized bed combustion processed samples than for sodium carbonate injection processed samples.
1. Introduction
Coal is the most abundant energy source in the world and therefore plays a crucial role in electric power generation. It is expected that the prominence of coal in the generation of electric power will continue in the future (Murarka, 1987). During the coal combustion process various by-products including, fly ash, bottom ash, and flue gases are generated. For example, in 1988 electric utilities in the USA generated approximately 90 million tons of coal ash. About 62% was fly ash, 23% was bottom ash and boiler slag, and 15% was flue gas desulfurization sludge. Of the total ash produced, only 20-25% was used in cement products, road bases, and asphalt. The remaining 75-80% was placed in surface impoundments and/or landfills.
The pH of fly ash can vary from 4.5 to 12.0 depending on the sulfur content of the parent coal, with high sulfur (eastern coals) generally producing acidic fly ash, and low sulfur (western coals) producing alkaline fly ash. Several studies have shown that certain trace elements (e.g., As, Cd, Se) in alkaline fly ash may become mobile and leach from disposal facility into soils and ground water (Cherry and Guthrie, 1977; Adriano et al., 1980; Kopsick and Angino, 1981; Adriano et al., 1982; Humenick et al., 1983; USEPA, 1988; Carlson and Adriano, 1993). However, the solubility and mobility of trace elements in fly ash disposal environments are not well understood (Mattigod et al., 1990; Eary et al., 1990).
The New Clean Air legislation enacted by the U.S. Congress mandated the reduction of SOX emissions from coal burning power plants. As a result, a variety of Clean Coal Technologies (CCT) such as furnace/duct sorbent injection, atmospheric fluidized bed combustion, spray dryer and wet flue gas desulfurization (FGD) scrubbing will likely be used. Such technologies are currently in various stages of commercial or experimental development in the United States.
Calcium (Ca2+) is one of the most abundant elements in fly ash, because it is not volatilized during the combustion process (Rai et al., 1987) and an alkaline sorbent (e.g., lime or sodium carbonate) is commonly used in the CCT process to remove SOX from flue gas. Furthermore, high temperatures of combustion process drive off C02 from carbonate phases (decarbonation). Consequently, the pH of aqueous extracts of CCT ash increases; this affects the solubility and mobility of trace elements.
With time, the pH of alkaline ash is expected to decrease with natural recarbonation (uptake of C02 by an alkaline ash). However, natural recarbonation is restricted by slow C02 input and reaction kinetics (Schramke, 1992). Such slow reactions may not prevent the mobility of trace elements from ash into disposal environments (i.e., soils and ground water). Nevertheless, reactions involving Ca2+ and C02 are expected to control the pH and solubility and mobility of trace elements in alkaline fly ash (Schramke, 1992). Despite the importance of these reactions, only a few studies have examined the effects a C02 treatment on alkaline solid wastes (i.e., fly ash and spent oil shale) (Reddy et al., 1986; Essington, 1989; Schramke, 1992). These studies either bubbled C02 through fly ash and spent shale slurries or aqueous solutions. However, such techniques are not practical for a typical coal burning power plant situation.
Recently, Reddy et al. (1991 and 1994) reported that reaction of C02 with alkaline spent oil shale and conventional coal combustion fly ash under slightly elevated pressures resulted in rapid precipitation of calcite, and thus reduced the concentration of extractable trace elements. In this study laboratory tests were conducted to determine the effects of pressurized C02 treatments on the chemistry of CCT ash samples. We collected different CCT ash samples and reacted them in a specially built chamber under different C02 pressure treatment conditions. Treated and untreated samples were subjected to X-ray diffraction (XRD) analysis and trace element extraction studies. Additionally, treated samples were subjected to calcite solubility studies. Results from these studies were used to determine the effects of pressurized C02 treatment on the pH and extractability of selected trace elements in CCT ash.
2. Materials and Methods
2.1. INITIAL CHARACTERIZATION
Three CCT ash samples (CCT-1, 2, and 3) were used in this study. The CCT-1 and CCT-2 ash samples were collected from a lime injection and atmospheric fluidized bed combustion processes, respectively. The CCT-3 ash samples were derived from a sodium carbonate injection process. Approximately, 4-5 kg of ash samples were collected either directly from the bag-house or electrostatic precipitators and were screened through a 0.25 mm mesh sieve. The sieved samples were subjected to the analysis of pH and total elemental concentrations.
The pH of the CCT ash samples was measured in a saturated paste with an Orion combination electrode. Samples were digested in nitric acid (6N) plus perchloric acid (60%). The solutions were then analyzed for total concentrations of Al, S, P, Ca, Fe, Mg, Mn, Cu, Cd, Zn, As, Se, and Mo by inductively coupled plasma optical emission spectrometry (ICP-OES). Sodium and K were measured by atomic absorption (AA). More details regarding these procedures are reported in Page et al. (1982).
2.2. C02 PRESSURE TREATMENT EFFECTS ON THE pH
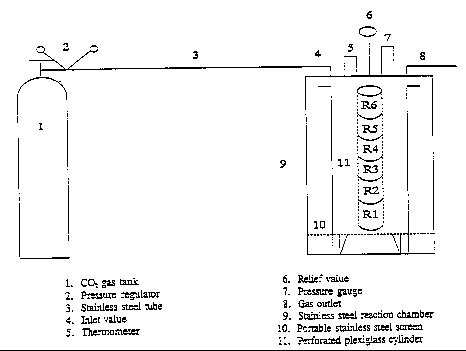
A stainless steel chamber, 30 cm in diameter by 60 cm in height (Fig. 1), was designed to react samples under different levels of C02 pressure (up to 125 psi). The reaction chamber was connected to a C02 tank (pure 99%, electronic instrument grade). A pressure gauge and a thermometer were installed on top of the chamber to monitor pressure and temperature, respectively. The gas outlet was connected to a fume hood.
A perforated plexiglass cylinder was designed, which consisted of six ring sample holder (11.87 cm in diameter by 7.5 cm in height) each separated by removable plexiglass disks (0.6 cm thick). This design assures effective diffusion of C02 through CCT ash samples during the treatment, since partial diffusion could lead to incomplete neutralization of alkalinity. The stainless steel chamber was wrapped in thermal wire along its length to monitor temperature. The chamber was covered with a foam insulation to reduce lateral heat flow losses to maintain a constant temperature.
Preliminary experiments were conducted to determine the efficiency of C02 diffusion through CCT ash samples. Different amounts of distilled-deionized H2O were added to the samples (on weight basis) and reacted under different levels of C02 partial pressure. After each preliminary treatment, samples were collected near the wall and at the middle of each ring to determine the efficiency of C02 diffusion through the samples.
Fig. 1. Experimental design of CO2 pressure process for CCT ash samples.
The efficiency of a C02 pressure treatment is dependent on variables such as moisture, C02 partial pressure, total pressure, temperature, and reaction time. Forty-six different treatment conditions were tested separately. These treatment conditions consisted of a range of variables (moisture = 5-50%, C02 partial pressure = 20-100%, pressure 50-125 psi, temperature = 25- 50° C, and reaction time = 24-72 hr). For each test, water was added to CCT ash samples on a weight basis, samples were then transferred to the plexiglass ring holders, and reacted under different conditions.
A part of each treated CCT ash samples was transferred to a plastic centrifuge tube to prepare 1:4 (solid:water) suspension and closed with caps. The pH of the suspension was measured within two hours and then monitored weekly for eighteen months to determine the effects of the C02 treatment on the long term stability of the pH. From 46 treatment conditions, final treatment conditions, which produced stable and lower pH's were selected. The CCT ash samples were then reacted with final treatment conditions and treated samples were used for subsequent experiments.
2.3. TREATMENT EFFECTS ON MAJOR MINERALS AND SOLUBILITY OF CALCITE
Treated and untreated CCT ash samples were subjected to XRD and calcium carbonate analyses. The XRD analysis was performed on randomly oriented slurried samples with a Scintag PAD V powder diffractometer using CuK a radiation. The XRD profiles obtained for each CCT ash sample were compared with Scintag software containing JCPDS (Joint Committee on Powder Diffraction Standards) files to determine major mineral phases. Calcium carbonate content was measured following the method of Nelson (1982). The % C02 absorbed by each CCT ash sample was calculated from the calcium carbonate content.
Treated samples (1:4 solid to water) were reacted on a mechanical shaker for determining the solubility of calcite (CaCO3) in aqueous extracts of CCT ash samples. After 7, 14, 21, and 28 day reaction time, sample suspensions were filtered through 0.45 mM Millipore filters under an Ar atmosphere to reduce the uptake of atmospheric C02 by leachates.
Each filtered leachate was divided into two subsamples. One was acidified to pH 5-6 with HN03. The other subsample was left unacidified. Acidified subsamples were analyzed for Ca, Mg, Al, Fe, Mo, Si (ICP-OES), Na, and K (AA). Unacidified subsamples were analyzed for pH, Cl-, F-, and SO42- (Ion Chromatography). The concentration of carbonate species was measured with C02 gas release method (Reddy et al., 1990).
The pH and total elemental concentrations of aqueous extracts were used as input to the MINTEQA2 model (Brown and Allison, 1992) to calculate Ca2+ and C032- activities. Calcite ion activity product (IAP) was calculated from ion activities and compared with the solubility product (Ksp=l0-8.48) of calcite (Plummer and Busenburg, 1982) to determine the solubility of calcite in aqueous extracts from treated CCT ash samples. We assumed that calcite IAPs within +- 0.50 log units of calcite Ksp represented equilibrium, and that calcite was a probable control on the concentration of Ca2+ andCO32-. The variation within that range is accounted for by the uncertainty of IAP estimates and Ksp measurements (Stumm and Morgan, 1981).
2.4. TREATMENT EFFECTS ON EXTRACTABLE ELEMENTS
The AB-DTPA (NH4HCO3 Diethylenetriaminepentaaceticacid) (Soltanpour and Schwab, 1977) extractant was used to extract trace elements from CCT ash samples. This extractant extracts both soluble (intensity fraction) and potentially soluble (capacity fraction) concentrations of trace elements from solid phases. The pH of the AB-DTPA extracting solution is maintained at 7.6 using either NH4OH or HCl which allows extraction of trace elements in alkaline materials. Several studies have used AB-DTPA (Folsom et al., 1981; Schwab et al., 1991; Reddy et al., 1994) to predict potential solubility and availability of trace elements from alkaline waste materials.
CCT ash samples were spiked with a multielement standard solution to a level of 50 mg/kg, each of Cd, Pb, Cu, Cr, As, Se, Ni, and B and were allowed to air dry. Duplicate spiked ash samples of 100 each were subjected to the C02 pressure treatment (spiked- treated) using final treatment conditions. Remaining spiked CCT ash samples were left without the C02 pressure treatment (spiked-untreated). The AB-DTPA procedure was used to extract Cd, Pb, Cu, Cr, As, Se, Ni, and B from CCT ash samples (unspiked and untreated; spiked and untreated; spiked and treated).
3. Results and Discussion
3.1. INITIAL CHARACTERIZATION
Initial characteristics of CCT ash samples are presented in Table I. All untreated CCT ash samples were high in Ca, K, Fe and low in Cd and Mo. CCT ash samples produced using lime and Na injection processes (i.e., CCT-1 & 3) were higher in Al, Mn, Cu and Pb contents than the one produced by atmospheric fluidized bed combustion process (CCT-2). However, the amounts of S, Fe, Zn and Mo were higher in the CCT-2 ash samples than in the CCT- I and 3 ash samples. Arsenic and Se concentrations in all CCT ash samples were below 20.0 mg/kg. These results show that CCT ash samples were different in their chemical composition. This may be due to the chemistry of the coal and the combustion process used in the power plants.
Saturated paste pH of untreated CCT ash samples ranged between 11.50 and 12.74 (Table I). Most CCT processes use an alkaline sorbent (e.g., calcium carbonate or sodium carbonate) to remove SOX from power plant flue gas. Additionally, high temperatures of the combustion process drive off C02 from carbonate phases, which results in the formation of alkaline earth oxide and silicate phases. These phases react with water, and as a result, the pH of aqueous extracts of CCT ash approach 12.0.
3.2. TREATMENT EFFECTS ON THE pH
Preliminary C02 diffusion experiments showed that C02 (g) circulated effectively through CCT ash samples during the pressure treatment. The pH of aqueous extracts of CCT ash samples dropped from 12.5 to an average value of 7.3. Measured pH values varied between 7.1 and 7.7 at the 12 sampling positions and the maximum variation was 0.20 within the rings and 0.60 between the rings. Regression analysis was performed to determine correlation coefficient (r2) values to establish the efficiency of the C02 diffusion through the plexiglass rings. Correlation coefficient (r2) between the pH at different positions within each ring was 0.99 and 0.98 between different rings. These results suggested that C02 was diffused effectively and the treatment effect on pH was nearly identical at all sampling positions.
TABLE I
Initial Characterization of CCT ash samples. Units are mg/kg
------------------------------------------------------------------------------------------------------------
Sample CCT-1 CCT-2 CCT-3
Process Lime Injection Atmospheric fluidized Na2CO3 injection
Bed combustion
------------------------------------------------------------------------------------------------------------
Al 109,500 300 77,400
Na 47,600 1,500 32,200
S 3,500 43,100 11,800
P 1,900 400 10,000
Ca 56,037 98,037 10,649
K 5,301 8,613 11,220
Mg 1,398 6,786 400
Fe 52,037 73,038 46,046
Mn 218 124 543
Cd 15 10 13
Cu 151 39 87
Pb 357 4 222
Ni 95 69 61
Zn 119 187 81
Mo 23 43 23
pH (saturated paste) 12.47 12.74 11.50
----------------------------------------------------------------------------------------------------------
Moisture was a limiting factor for all CCT ash samples for rapid chemical stabilization. High levels of pressure and % C02 were also closely related to the pH of some CCT ash samples. A significant drop in the pH was measured immediately after removing samples from the treatment chamber. Over time, some CCT ash samples maintained their low treatment pH values, while others returned to values close to those measured before treatment.
Final treatment conditions that significantly reduced the pH of each CCT ash sample were different. The CCT-1 ash samples stabilized at lower pH values than the CCT-2 and 3 samples. The methods used in the CCT process (i.e. calcium carbonate or sodium carbonate) were critical in determining the effectiveness of the C02 treatment with respect to pH. Samples processed using lime injection (CCT-1 ash samples) responded more rapidly to the treatment with respect to pH, than those produced under atmospheric fluidized bed combustion (CCT-2 ash samples) or sodium carbonate injection (CCT-3 ash samples).
Thirteen treatment conditions produced a significant stable drop in the pH values of CCT-1 ash samples (Table II). A drop of between 3.89 and 5.42 +- 0.10 pH units was achieved by different combinations of treatment variables. Treatments 1 through 6 produced an average pH drop of 4.47. These results show that the % moisture level is the most sensitive treatment variable. Increasing the % moisture from 15% to 20%
TABLE II
Effects of C02 treatment variables on the pH of lime injection ash samples (CCT-1).
The pH before treatment = 12.47
| No. | Moisture % |
Press. (psi.) |
C02 (%) |
Temp. (° C) |
Time (hr) |
pH-A | pH-F | pH-B | D pH |
| --------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | |||||||||
| 1 | 15 | 75 | 100 | 25 | 48 | 7.77 | 8.58 | +0.81 | 3.89 |
| 2 | 15 | 100 | 100 | 25 | 48 | 7.98 | 8.34 | +0.36 | 4.13 |
| 3 | 20 | 50 | 100 | 25 | 24 | 7.74 | 8.13 | +0.39 | 4.34 |
| 4 | 20 | 75 | 100 | 25 | 24 | 7.89 | 7.65 | -.024 | 4.82 |
| 5 | 20 | 100 | 100 | 25 | 48 | 7.67 | 7.55 | -0.12 | 4.92 |
| 6 | 20 | 125 | 100 | 25 | 48 | 7.68 | 7.74 | -0.06 | 4.73 |
| 7 | 20 | 100 | 50 | 25 | 48 | 6.81 | 8.04 | +1.23 | 4.43 |
| 8 | 20 | 100 | 20 | 25 | 48 | 8.23 | 8.15 | -0.08 | 4.32 |
| 9 | 20 | 50 | 20 | 50 | 48 | 7.44 | 7.29 | -0.15 | 5.18 |
| 10 | 20 | 50 | 50 | 25 | 24 | 7.88 | 7.50 | -0.38 | 4.97 |
| 11 | 20 | 100 | 100 | 45 | 24 | 8.20 | 7.18 | -1.02 | 5.29 |
| 12 | 20 | 75 | 75 | 50 | 48 | 7.45 | 7.76 | +0.31 | 4.71 |
| 13a | 20 | 75 | 75 | 25 | 24 | 7.45 | 7.05 | -0.40 | 5.42 |
(treatments 1 & 2 vs. 3 & 4) produced lower pH values under less pressure and reaction time. Further, increasing pressure and reaction time (treatments 5 & 6 vs. 4) with 20% moisture did not produce a significant change in the pH (D pH = +- 0.10).
Treatments 7, 8, 9 and 12 show that increasing temperature with 20% moisture under lower %CO2 and pressure produced a further reduction in the pH. No significant differences were found between treatments 4 and 12 (D pH = +- 0.10), but treatment 9 produced a D pH of 5.18 with low CO2 pressure. Treatments 4, 5, 10, 11 and 13 averaged a D pH drop of 5.08 with less reaction time, pressure and %CO2. However, treatment 13 was the most effective and produced the largest pH drop using, medium levels of %CO2 and pressure. Therefore, these conditions were selected as a final treatment for CCT- 1 ash samples.
Initial results from different CO2 pressure treatment experiments for CCT-2 ash showed that pH of these samples did not stabilize under any treatment conditions. Moisture addition to CCT-2 ash samples, before CO2 pressure treatment, increased the temperature (approximately 120° C) of the samples due to the dissolution of oxides. This resulted in the consumption of moisture (approximately 50 to 60%) by the formation of
TABLE III
Effects of CO2 treatment variables on the pH of atmospheric fluidized bed combustion
ash samples (CCT-2). The pH before treatment = 12.74
---------------------------------------------------------------------------------------------------------------------------------------------------------------------
| No. | Moisture % |
Press. (psi.) |
C02 (%) |
Temp. (° C) |
Time (hr) |
pH-A | pH-F | pH-B | D pH |
| ------------------------------------------------------------------------------------------------------------------------------------------------------------------------ | |||||||||
| 1 | 25 | 100 | 100 | 25 | 24 | 8.03 | 9.50 | +1.47 | 3.24 |
| 2 | 25 | 100 | 100 | 25 | 48 | 8.05 | 9.55 | +1.50 | 3.19 |
| 3 | 25 | 125 | 100 | 25 | 48 | 7.73 | 9.50 | +1.77 | 3.24 |
| 4 | 25 | 100 | 100 | 25 | 72 | 8.05 | 9.58 | +1.53 | 3.16 |
| 5 | 40 | 100 | 100 | 25 | 96 | 7.74 | 9.52 | +1.78 | 3.22 |
| 6 | 50 | 100 | 100 | 25 | 96 | 7.66 | 9.42 | +1.76 | 3.32 |
| 7a | 50 | 100 | 100 | 45 | 24 | 8.55 | 9.34 | +0.79 | 3.40 |
hydrated precipitates. The addition of moisture, lost during the formation of hydrated precipitates, before each treatment was a critical factor in obtaining a stable pH drop for CCT-2 ash samples.
Treatments 1 through 7 (Table III) produced a significant drop in the pH values for the CCT- 2 ash samples. The lower stable pH values measured after the treatments ranged from 9.58 to 9.34, with an average D pH of 3.25. No significant differences were found between treatments 1, 2, 3 and 4. Increasing the % moisture to 40 and 50 under room temperature (treatments 5 and 6) showed no further drop in the pH. However, increasing temperature to 45 ° C reduced the pH from 12.74 to 9.34 in 24 hr (treatment 7), and this treatment was selected for CCT-2 ash samples.
Treatments 1 through 4 (Table IV) produced an average D pH of 2.30 for CCT-3 ash samples. Initial pH values around 8.50 were obtained by different treatment conditions. However, these initial pH values were stabilized near 9.20 in most treatments, and treatment 2 was selected for CCT-3 ash samples. The high buffering capacity and the apparent slow dissolution of silicate minerals, and also the low concentration of Ca as compared to %Na, might have prevented attainment of a larger drop in the pH of CCT-3 ash samples.
3.3. TREATMENT EFFECTS ON MAJOR MINERALS AND SOLUBILITY OF CALCITEThe XRD analysis (Table V) suggested that CCT ash samples consisted largely of quartz, silicates, amorphous phases, and calcium oxide. Formation of these phases in CCT ash samples require high temperatures (i.e., >1500
TABLE IV
Effects of CO2 treatment variables on the sodium carbonate injection ash samples (CCT-
3). The pH before treatment = 11.50
| No. | Moisture % |
Press. (psi.) |
C02 (%) |
Temp. (° C) |
Time (hr) |
pH-A | pH-F | pH-B | D pH |
| ------------------------------------------------------------------------------------------------------------------------------------------------------------------ | |||||||||
| 1 | 10 | 100 | 20 | 25 | 48 | 8.72 | 9.14 | +0.42 | 2.36 |
| 2a | 10 | 75 | 75 | 25 | 24 | 8.71 | 9.16 | +0.45 | 2.34 | 3 | 10 | 100 | 50 | 25 | 48 | 8.49 | 9.21 | +0.72 | 2.29 | 4 | 10 | 100 | 100 | 45 | 24 | 8.55 | 9.28 | +0.73 | 2.22 |
TABLE V
Effects of CO2 treatment conditions on %CaCO3 equivalent,
%CO2, and mineral transformations. N.D. = not detected
| Sample | %CaCO3 equivalent |
%CO2 | Major mineral peaks |
| ----------------------------------------------------------------------------------------------------------------------------------------- | |||
| CCT-1 Untreated | N.D. | N.D. | quartz, silicates |
| CCT-1 Treated | 2.82 | 0.62 | calcite, gypsum, quartz, mullite |
| CCT-2 Untreated | N.D. | N.D. | quartz, calcium oxide, amorphous phases |
| CCT-2 Treated | 4.48 | 0.99 | calcite, gypsum |
| CCT-3 Untreated | 3.38 | 0.74 | quartz, amorphous phases |
| CCT-3 Treated | 4.57 | 1.01 | quartz, calcite |
° K). Such temperatures are usually attained during the combustion process of coal. The %CaCO3 equivalent and %CO2 (Table V) were increased significantly after the CO2 pressure treatment. Both CaCO3 and CO2 were not detected in untreated CCT-1 and 2 ash samples, but both were increased upon CO2 pressure treatment. This increase in carbonate content suggests the probable dissolution of oxides and silicates and the precipitation of calcium carbonates. The XRD analyses indicated the presence of calcite in treated CCT ash samples (Table V). The results in Table V also show that CO2 treatment caused the precipitation of gypsum (CaSO4-2H2O) in CCT ash samples.
TABLE VI
Saturation index (SI) for calcite in aqueous
extracts of CO2 treated CCT ash samples
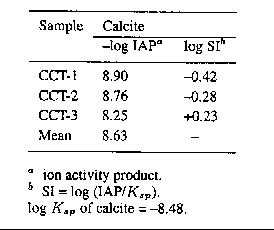
Chemical data from 7, 14, 21, and 28 day calcite solubility studies suggested that no significant changes were found between 21 and 28 days. Therefore, the chemical data of 28 days reaction time were used to determine the saturation index of calcite in aqueous extracts of treated CCT ash samples (Table VI). The log IAPs for treated CCT samples showed near saturation with respect to calcite (a mean value of -8.63). These results support the XRD findings with treated CCT samples. Long term leaching experiments suggested that calcite could precipitate in weathered fly ash (Dudas, 1981). Rai et al. ( 1987) also reported similar results.
3.4. TREATMENT EFFECTS ON EXTRACTABLE TRACE ELEMENTS
The CO2 pressure treatment effects on extractable trace elements were clearly observed from the results of the AB-DTPA extraction procedure (Table VII). A reduction in the concentrations of extractable trace elements was obtained in spiked plus CO2 treated CCT ash samples. For instance, extractable arsenic (As) concentrations were decreased from 3.40 to 0.10 mg/L for CCT-1 ash samples. The decrease in extractable As from 0.35 to 0.10 and 3.70 to 1.15 mg/L was observed for CCT-2 and CCT-3 ash samples, respectively. The CO2 pressure treatment was found most effective for CCT-1 ash samples (lime injection process), followed by CCT-2 (atmospheric fluidized bed combustion process), and CCT-3 (sodium carbonate injection process) ash samples in reducing the concentration of extractable trace elements.
A possible explanation for the decrease in the concentration of extractable trace metals (e.g., Cd, Pb) is the precipitation of metal carbonates. In CCT ash samples, these metals are probably present as oxides and/or hydroxides (e.g., CdO, Pb(OH)2) due to the combustion process. When CCT ash samples were reacted under CO2 pressure, soluble oxides and/or hydroxides were probably converted to carbonates (e.g., CdCO3, PbCO3). This causes a reduction in the concentration of extractable metals because carbonate phases are less soluble than oxides or hydroxides between pH 8.0 and 9.0 (Lindsay, 1979).
TABLE VII
Effects of final CO2 treatment conditions on extractable trace elements. Units are
mg/L
--------------------------------------------------------------------------------------------------------------------------------------------------------------------
| I.D a | Cd | Pb | Cu | Cr | As | Se | Ni | B |
| --------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ||||||||
| 1-C | 0.01 | 0.10 | 1.42 | 0.32 | 0.75 | BD | 1.70 | 27 |
| 1-B | 5.35 | 4.55 | 12.82 | 0.17 | 3.40 | 3.75 | 2.25 | 57 |
| 1-A | 0.04 | 0.50 | 2.65 | 0.02 | 0.10 | 0.65 | 0.20 | 19 |
| %Dropb | 99 | 89 | 79 | 88 | 97 | 82 | 90 | 66 |
| 2-C | 0.01 | 0.56 | 0.09 | 0.43 | 0.03 | 0.03 | 0.17 | 26 |
| 2-B | 4.50 | 2.65 | 1.73 | 2.05 | 0.35 | 2.15 | 0.32 | 15 |
| 2-A | 0.45 | 1.15 | 1.65 | 1.10 | 0.10 | 0.50 | 0.30 | N.A. |
| %Drop | 90 | 56 | 4 | 46 | 71 | 76 | 6 | N.A. |
| 3-C | 0.09 | 0.50 | 2.90 | 0.75 | 2.65 | 4.25 | 0.02 | 176 |
| 3-B | 3.96 | 5.85 | 5.21 | 0.33 | 3.70 | 12.45 | 2.15 | 192 |
| 3-A | 3.10 | 3.30 | 3.91 | 0.08 | 1.15 | 8.20 | 1.81 | 158 |
| %Drop | 21 | 43 | 24 | 75 | 68 | 34 | 15 | 17 |
A reduction in the concentration of extractable As, B, Cr, and Se is probably attributable to an increased sorption of these elements by iron oxides at lower pH values because CCT ash commonly contains iron oxides (see Table I). It is well established that by decreasing the extractable concentration of a given element, the mobility of that element will also be reduced.
Theis and Wirth (1977) reported that release of trace elements (e.g., As, Cd, Cr, Cu, Pb, Ni, and Zn) from coal combustion ash is strongly related to the pH. Results from their studies also suggest that as pH decreases from 12 to 9 these elements become less soluble in fly ash due to sorption and precipitation processes. However, release of trace elements from coal combustion ash increased significantly, when pH dropped to 6 or below (Theis and Wirth, 1977). These results show that trace elements in coal combustion ash attain their lowest solubility between the pH of 9 and 6. Rai et al. (1987) also reported similar results.
Thus, observed reduction in the concentrations of extractable trace elements in C02 treated CCT ash samples is probably due to the mineral phases formed as pH is reduced through the consumption of C02, which in turn enhanced both sorption and precipitation processes of trace elements. The C02 pressure treatment described here has, under laboratory conditions, demonstrated its potential to reduce the concentration of extractable trace elements (As, Cd, Pb, and Se) in different CCT ash samples. This could reduce the potential mobility of these elements in C02 treated CCT ash.
4. Conclusions
The C02 pressure treatment produced a significant drop in the pH of CCT ash samples. The C02 treatment also caused the dissolution of oxide and silicate phases and the precipitation of CaCO3 and CaSO4 * 2H2O phases. Clean coal technology process used in each power plant was an important factor in determining the efficiency of the C02 treatment for chemical stabilization of CCT ash. Lime injection played a key role by supplying the Ca needed to enhance the rapid precipitation of CaCO3 and CaSO4 phases in CCT ash samples. Additionally, moisture, C02 concentration, and pressure were also found to affect the efficiency of the C02 treatment process for CCT ash.
The C02 pressure treatment effectively decreased the concentration of extractable trace elements in CCT ash samples produced from lime injection and atmospheric fluidized bed combustion processes. Since this process uses C02, which can be obtained from the combustion process itself (i.e., flue gas), it has the potential to concomitantly reduce C02 (so-called greenhouse gas) emissions from the coal combustion process. Further research is needed to evaluate the cost and economic viability of the C02 pressure process under field conditions.
Acknowledgements
The authors extend their appreciation to the Land and Water Quality Studies Program, Environment Division of the Electric Power Research Institute for funding this research. We thank Drs. Ishwar P. Murarka and John W. Goodrich-Mahoney, Electric Power Research Institute for reviewing the initial manuscript. We also thank Srinivas Dhanam for his help in various aspects of the research and the Power Plants for providing CCT ash samples.
References
Adriano, D.C., Page, A.L., Elseewi, A.A., Chang A.C., and Straughan, I.R.: 1980, J. Environ. Qual. 9, 333-334.
Adriano, D.C., Page, A.L, Elseewi, A.A., and Chang, A.C.: 1982, J. Environ. Qual. 11, 197-203.
Brown, D.S., and Allison, J.D.: 1992, MINTEQA2, An Equilibrium Metal Speciation Model. Environmental Protection Agency, Environmental Research Laboratory, Athens, GA.
Carlson, C.L., and Adriano, D.C.: 1993, J. Environ. Qual. 22, 227-247.
Cherry, D.S., and Guthrie, R.K.: 1977, Water Resour Bull. 13, 1227-1235.
Dudas, M.J.: 1981, Environ. Sci. Technol. 15, 840-843.
Eary, L.E., Rai, D., Mattigod, S.V., and Ainsworth, C.C.: 1990, J. Environ. Qual. 19, 202-214.
Essington, M.E.: 1989, Environ. Geol. Wat. Sci. 13, 59-66.
Folsom Jr., B.L., Lee, C.R., and Bates, D.L.: 1981, Influence of Disposal Environment on Availability and Plant Uptake of Heavy Metals in Dredged Material, Army Engineers Waterways Experiment Station Report No. WES/tr/El-81012.
Humenick, M.J., Lang, M., and Jackson, K.R: 1983, J. Water Pollut. Control Fed. 55, 310-316.
Kopsick, D.A., and Angino, E.E.: 1981, J. Hydrol. 54, 341-356.
Lindsay, W.L.: 1979, Chemical Equilibria in Soils, John Wiley & Sons, Inc. New York.
Mattigod, S.V., Rai, D., Eary, L.E., and Ainsworth, C.C.: 1990, J. Environ. Qual.19, 188-201.
Murarka, I.P.: 1987, Solid Waste Disposal and Reuse in the United States, Boca Raton, Florida. CRC Press, Inc.
Nelson, R.E.: 1982, 'Methods of Soil Analysis', part 2. Chemical and Microbiological Properties. Agronomy Monograph No. 9 (2nd Edition), pp. 181-197.
Page, A.L., Miller, R.H., and Keeney, D.R.: 1982, 'Methods of Soil Analysis', part 2. Chemical and Microbiological Properties. Agronomy Monograph No. 9 (2nd Edition).
Plummer, L.N., and Busenberg, E.: 1982, Geochim. Cosmochim. Acta. 46,1011- 1040.
Rai, D., Ainsworth, C.C., Eary L.E., Mattigod S.V, and Jackson, D.R.: 1987, EPRI EA-5176.
Reddy, K.J., Lindsay, W.L., Boyle, F.W. and Redente, E.F.: 1986, J. Environ. Qual. 15,129-133.
Reddy, K.J., Lindsay, W.L., Workman, S.M., and Drever, J.I.: 1990, Soil Sci. Soc. Am. J. 54, 67-71.
Reddy, K.J., Drever, J.I., and Hasfurther, V.R.: 1991, Environ. Sci. Technol. 25, 1466-1469.
Reddy, K.J., Gloss, S.P., and Wang, L.: 1994, Water Research. 28, 1377-1382.
Schramke, A.J.: 1992, Applied Geochemistry. 7, 481-492.
Schwab, A.P., Tomecek, M.B., and Ohlenbusch, P.D.: 1991, Water, Air, and Soil Pollut. 57-58,297-306.
Soltanpour, P.N., and Schwab, A.P.: 1977, Comm. Soil Sci. Plant Anal. 8, 195- 207. Stumm, W., and Morgan, J.J.: 1981, Aquatic Chemistry, Wiley-Interscience, New York.
Theis, T.L., and Wirth, J.L.: 1977, Environ. Sci. Technol. 11, 1096-1100.
U.S. Environmental Protection Agency.: 1988, Waste from the Combustion of Coal, EPA Report 530-SW-88-002.
Water Resources Publications List
Water Resources Data System Library |
Water Resources Data System Homepage