| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| John C. Adams | December 1985 WWRC-85- 19 |
Final Report
(Grant No. 2-92464)
Submitted to
Wyoming Water Research Center
University of Wyoming
Laramie, Wyoming
Submitted by
John C. Adams
Division of Microbiology and
Veterinary Medicine
University of Wyoming
Abstract
Blooms of algae tentatively identified as Asterionella and Anabaena occurred at times when tastes and odors developed in the surface water source of Laramie's drinking water during the summer of 1984. Actinomycetes were found in Laramie's surface water source but did not appear to be numerous enough to be responsible for the tastes. Filamentous algae did not become numerous enough to present a problem at the wastewater treatment facility during the summer of 1984. Selected bacteria were enumerated through the surface water treatment plant during the summer of 1984. This plant reduced numbers of bacteria from raw to finished water by 99.99 percent. Fecal coliforms survived for three months in material taken from the sedimentation basins. In some cases R2A medium was the superior medium for determining the heterotrophic plant count.
Introduction
This project was developed from an inquiry to the PI from the City of Laramie Water Department asking for help concerning the identification of the cause of a taste and odor problem that recently seemed to be occurring each summer. Secondly, there was the potential for a problem of clogging the infiltration-percolation beds of the wastewater treatment facility by filamentous algae and help was requested with this problem.
The taste and odor problem could have been due to either algal growth or to the growth of actinomycetes. Actinomycetes are bacteria which may produce two chemical compounds which have been implicated in taste and odor problems of water. These are geosmin and 2 methyl-isoborneol. These two taste compounds are also produced by various algae. It was possible that the actinomycetes were growing in the treatment plant.
This project was undertaken primarily to determine what caused the tastes and odors and secondarily, to gain some information concerning the bacteriology of Laramie's drinking water.
Requirements of the Project (Objectives)
Methodology
Studies concerned with algal growth were done by filtering various amounts of water through a Millipore HA gridded filter with a pore size of 0.45 micrometers. The filters were placed upon a glass slide and observed at 100X magnification. The number of algal cells per square of the grid was determined. In some cases total cells were counted while in other cases only the predominant organism was counted. Studies with the lagoons were only concerned with the enumeration of filamentous algae. At the water plant 100 mls of raw water were routinely filtered. Occasionally samples of Lake Sodergren were filtered. Five ml samples from the lagoons were filtered. Identification of algae was done using the 15th edition of Standard Methods for the Examination of Water and Wastewater.
Methodologies used for studying the bacteriology of Laramie's drinking water are detailed in the attached thesis.
Results
Presented in Table 1 are the results of approximately weekly samples taken to determine the presence of algae in the raw water. Very few algae were seen until the end of July. At the time of taste development the total algae were ignored and the predominant type counted. The first taste problem, which lasted for a short time, corresponded to an increase in the number of Asterionella up to eight colonies per square of filter. This organism is listed in Standard Methods as a taste causing organism. A slight taste then persisted through the bloom of an organism tentatively identified as Ulothrix. The real taste problem started with the appearance of an organism tentatively identified as Anabaena. The taste was present when as few as two filaments per square of filter were seen and got worse as the filaments increased in size and number. It was finally decided by the city to shut the plant down in early September. The numbers of this organism in the raw water reached 40-50 filaments per square of filter while they were as high as 80 per square in the surface water of Lake Sodergren.
Filamentous algae did not grow in the sewage lagoons to any extent during the summer of 1984, thus no problem was encountered and no data is available. The predominant algae in the sewage lagoons were identified as Chlorella and Scenedesmus with Chlorella being dominant in May and June while Scenedesmus was most numerous in July and August.
For the results of the bacterlological studies see the attached thesis.
Table 1. Algae in Laramie's Raw Water During the Summer of 1984 Unidentified Identified Organisms --------------------- -------------------------------------------- Green or blue Date green algae Diatoms Asterionella Ulothrix Pandorina Anabaena Taste -------------------------------------------------------------------------------- 5-22 1* a+ a a a a No 6-6 a a a a a a No 6-13 a a a a a a No 6-20 2 a a a a a No 6-26 a a a a a a No 7-5 a a a a a a No 7-12 1 a a a a a No 7-19 2 a a a a a No 7-25 - =/ - 8 a 1-2 a Yes 7-26 - - 1.4 a 1.6 a Yes 8-6 many - a 80 a a Slight 8-21 - - a 10 1 2 Slight 8-22 - - a 10 1 2 Slight 8-23 - - a 10 1 4 Yes 8-24 - - a - a 4.4 Yes 8-25 - - a Anabaena filaments 4 Yes were longer than previous days 8-30 - - a - - 35 Yes 9-5 Plant shut down very bad taste - 47 Yes 9-11 - - - - - 40-50 Yes 9-13 - - - - - 40-50 Yes -------------------------------------------------------------------------------- * - number per square of gridded membrane filter + - less than one per square of gridded membrane filter =/- not determined
Conclusions
Recommendations
Appendix
Kiessling, William M., Microbiological Studies of Laramie's Drinking Water, M.S., Department of Microbiology and Biochemistry, December, 1985.
Selected bacterial populations were enumerated from raw and finished drinking water in Laramie, Wyoming. In certain cases a higher percentage of the total heterotrophic bacteria were recovered on R2A than on modified Henrici agar. The number of actinomycetes could not explain the appearance of tastes and odors. Sulfate reducing bacteria were enumerated. The predominant organisms varied with the site sampled.
MICROBIOLOGICAL STUDIES OF
LARAMIE'S DRINKING WATER
by
William M. Kiessling
A Thesis
Submitted to the
Department of Microbiology and Biochemistry and
The Graduate School of the University of Wyoming
In Partial Fulfillment of the Requirements
for the Degree of Master of Science
University of Wyoming
Laramie, Wyoming
December, 1985
ACKNOWLEDGEMENT
I would like to thank Dr. John C. Adams whose patience, advise, technical assistance, and friendship were invaluable. Appreciation is also extended to Drs. Leroy R. Maki, Daniel R. Caldwell and Quentin D. Skinner for their helpful criticism. Funding was provided through the Wyoming Water Research Center.
To my parents, William and Helen Kiessling, whose encouragement was also present, this thesis is dedicated.
The assistance and cooperation of the Laramie Water Department personnel, especially Wes Bressler and Rick Schneider, is appreciated.
INTRODUCTION
Domestic drinking water, whether supplied from ground water sources such as springs and wells, or surface water sources from lakes and rivers, is usually treated in some manner to remove or kill microorganisms. The presence of total coliforms, fecal coliforms and fecal streptococci in potable water has been used widely as an indication of the general water quality and the efficiency of treatment. The heterotrophic bacterial population in drinking water however, often exceeds that of the coliform group. Previous work has shown that enumeration of both heterotrophic bacteria and the coliform group is required to adequately evaluate the bacteriological quality of water (20).
Monitoring the heterotrophic population is necessary because high plate counts may indicate the presence of non-fecal opportunistic pathogens (20). Also, high numbers of heterotrophic bacteria in water have been shown to interfere with the detection of coliform bacteria (20,34) thus resulting in the potential for coliforms in contaminated water remaining undetected by routine analysis. Some heterotrophic bacterial strains have been associated with the production of tastes and odors in water (63,64,70), and the deterioration of distribution systems as the result of formation of encrustations or tubercles on the interior of pipelines (1,37,40). These deposits provided habitats which protect bacteria from residual chlorine (70), cause internal corrosion (10,53), and reduce the water capacity of pipelines (70). Sulfate reducing bacteria have been shown to be of particular importance in corrosion problems (10,40,53).
This study was initiated to determine: 1) if actinomycetes at some point in the treatment process were responsible for a taste and odor problem that was occurring in Laramie's drinking water. 2) isolate and identify bacteria from the distribution system and determine if particular organisms originated in the different water sources and were found at specific points in the distribution system. 3) determine the numbers of sulfate reducing and other types of bacteria at various points in the surface water treatment plant.
LITERATURE REVIEW
Interest in the quality of domestic drinking water supplies has grown in recent years because of the increased demands for water, the enactment of the Safe Drinking Water Act of 1974 and the waterborne epidemics which have occurred in the United States in past years. The goal of the Safe Drinking Water Act of 1974 was to enhance the quality of drinking water in the United States by imposing stricter water quality regulations (39).
In the period from 1971-1978, 224 waterborne outbreaks were reported in the United States (13,24). Contamination of the distribution system and treatment deficiencies were reported as the major causes of the outbreaks from municipalities (13). More outbreaks were reported from nonmunicipal sources than were reported from municipalities. However, most of the reported causes of illness were from municipal systems (14). A higher number of outbreaks occurred from municipal systems with ground water sources than from systems with surface water sources (14). McCabe et al. (47) reported numerous instances of poor quality water originating from ground water sources. The majority of these sources were unprotected or inadequately treated.
Locating a domestic source of water as free from contamination as possible is desirable, but people do not always settle in areas where water of this quality is available. Therefore, water must be treated to make it safe for human consumption. The basic treatment of water involves the removal of organisms by filtration or by killing them with chlorine (18). Galney and Lord (18) stated that the important treatment procedures were storage or sedimentation of water without coagulation, coagulation followed by sedimentation and rapid sand filtration, and chemical disinfection by chlorination. They reported that coagulation and sedimentation removed 50 to 99 percent of the bacteria in the raw water. The average removal was around 80 percent. For a coagulant to perform adequately it must form a water insoluble floe which traps bacteria and removes them from the water either by sedimentation or by filtration (18). Aluminum sulfate (alum) has been used widely for this purpose (18). The survival and possible release of organisms, including pathogens, from the floe is of recent public health concern (9).
Bulson et al. (9) studied the removal of bacteria from a lake treated with alum. Application of alum to Liberty Lake in Washington effectively removed 95 percent of the total culturable bacterial population. Ninety percent of the fecal coliforms and 70 percent of the fecal streptococci were removed from the water column and concentrated in the alum floe at the bottom of the lake. Laboratory experiments in this study showed that the concentration of Escherichia coli was reduced in the water column from 1.5 x 108 cells per 100 ml to 1.5 x 103 cells per 100 ml at 30 h after flocculation. E. coli cells were not released from the flocculated material to the water column and die-off was reported at a greater rate than was observed for unflocculated cells. The release of bacteria from the floe by use of a blender has been reported (48). Blending times from 2 to 18 seconds resulted in no significant cell injury, however, blending exceeding 18 seconds was found to cause cell damage (9).
Rapid sand filtration has been shown to remove 60 to 90 percent of the bacteria applied to the filter with an average around 80 percent (18). Gainey and Lord (18) reported that some bacteria may pass through the filter and cautioned that all water processed by rapid sand filtration must be chlorinated to protect the consumer.
Chlorine is an effective disinfectant in water, however, the specific action of chlorine on bacteria remains unclear (18). Green and Stumf (23) reported that chlorine inhibits glucose oxidation by the cell. Knox et al. (32) indicated that chlorine affected the sulfhydryl groups of enzymes responsible for carbohydrate metabolism. Venkobachar et al. (72) examined the effects of chlorine on dehydrogenases and Benarde et al. (5) suggested that protein synthesis was the target upon which chlorine acted. Camper and McFeters (12) reported that chlorine affects the transport of extracellular nutrients across the cell membrane.
The ability of bacteria to repair chlorine injury has been debated. Some authors have indicated that the viable cells enumerated after chlorination resulted from the proliferation of the surviving population (19,28) while others have reported that actual repair of chlorine injured cells had occurred (12,46,50). The detection of these injured cells in water is important for an accurate assessment of water quality (46) because some might be pathogens which could recover in the human body.
There is much literature on detection and enumeration procedures for various types of bacteria in water and the efficiency of these methods (6,7,16,17,22,27,29,30,41,43,48,49,52,55,56,62,67,68). Reliable detection methods are necessary because high numbers of coliform bacteria can indicate the presence of pathogens (34). High non-coliform counts in water supplies have been shown to suppress the detection of coliform bacteria (20,34). Geldreich et al. (2) reported that the frequency of total and fecal coliform detection decreased as the heterotrophic plate count bacteria exceeded 1,000 per ml. Larnka et al. (34) reported similar results when heterotrophic plate counts increased above 500 per ml. Medium composition, incubation temperature and incubation time have a major influence on how successfully the bacterial population was enumerated from nonchlorinated and chlorinated water (17,48).
The standard plate count has been the method generally used to enumerate bacteria from water (56). The standard plate count procedure employs the pour plate technique using either plate count agar or tryptone glucose yeast extract agar incubated at 30°C for 48 hours (2). Many investigators have reported that the pour plate procedure yields lower counts than the streak or spread plate method (31,49,55,71). Means et al. (49) studied the recovery of bacteria obtained from unchlorinated water supplies to the city of Garden Grove and chlorinated water obtained from Irvine, California. Results from this study showed that the standard plate count procedure underestimated the heterotrophic bacterial population by 53 percent in the unchlorinated water system and 40 percent in the chlorinated system when compared to the spread plate counts determined on R2A medium. Reasoner and Geldreich (56) have shown that the R2A medium was superior to plate count agar in enumerating heterotrophic bacteria from treated water. Modified Henrici agar and R2A agar contain diverse carbon and energy sources which may explain the increased recovery of bacteria as compared to standard plate count agar (56). R2A agar was the medium of choice to enhance the pigment production of heterotrophic bacteria (49,56). Reasoner and Geldrich (56) found that an incubation time of 7 days at 35°C resulted in R2A spread plate counts five-fold greater than counts obtained with plate count agar plates from finished water. Optimum counts were obtained when plates were incubated at 20°C for 7 days.
Studies have shown that slow-growing pigmented, water bacteria, which include species of Cytophaga, Aeromonas, Pseudomonas, and Flavobacterium, often survive chlorination and can be found in the distribution system (21,25). Herman (25) found that 78 percent of 150 drinking fountains studied had been contaminated with slow-growing pigmented water bacteria. Sink faucets, water baths and humidifying units were also found to be contaminated by these organisms. Matsen (44) reported that this group of organisms, with the exception of Pseudomonas aeruginosa, is seldom considered to be major pathogens and are usually only associated with hospital acquired infections. Healthy individuals did not appear to suffer from low-level contaminated water (21), however, the hazard to debilitated or compromised individuals is one which should not be ignored (25).
A major group of organisms found in fresh water is the fluorescent bacteria (26). Skinner et al. (65) have shown that stream systems within a high mountain watershed in Wyoming contain around 1.0 x 104 fluorescent bacteria per 100 ml. One member of this group, P. aeruginosa, was a potential pathogen (15). Drake (15) has shown that the growth of P. aeruginosa is possible over a temperature range of 30°C and that the organism can tolerate wide pH values. The presence of this organism may also be evidence of recent fecal contamination (15,26).
The consumers basis for evaluating water quality includes clarity and the absence of any tastes and odors (42). Drinking water supplies may periodically develop taste and odor problems from various types of organisms (64). One group of microorganisms, the actinomycetes, have been linked to taste and odor problems in drinking water (51,63). Morris et al. (51) reported that the musty tastes and odors from Cedar River, Iowa, were caused by certain metabolites of actinomycetes. Romano and Safferman (58) found that soil forms were capable of producing musty tastes and odors in surface waters, thus indicating the potential for contamination caused by surface runoff. Silvey (64) made the observation that taste and odor problems in drinking water were widespread across the United States and suggested that problems due to actinomycetes could occur in any area of the nation when the average temperature is above 20°C for 3 months.
Microorganisms have not only been found to cause taste and odor problems but also have become linked with the deterioration of distribution pipelines (1,10,37,40,53,70). The deterioration was in the form of encrustations or tubercles on the interior of water mains. Diatoms, algae, actinomycetes and sulfate reducing bacteria have been shown associated with these deposits (1,10,70). Tuovinen et al. (70) examined deposits from the Columbus, Ohio, distribution system and found that the interior of the tubercle provided a suitable habitat for anaerobic sulfate reducing bacteria. Butlin et al. (10) isolated approximately 1,000 sulfate reducing bacteria per gram from the interior of tubercles. Microorganisms within the tubercle were protected from the action of chlorine (70). Tuovinen et al. (70) reported that tubercles can become dislodged from the pipeline during periods of high flow. This indicated the possibility for very high heterotrophic plate counts from water within the pipe system when organisms were released in this manner (53). O'Connor et al. (53) found that the growth of sulfate reducing bacteria could produce areas of low pH at the pipe surface. The sloughing of groups of bacteria could allow oxygen to reach a portion of the interior pipe surface forming an electrode system which accelerates the corrosion process.
The use of granular activated carbon (GAC) filtration has been shown to reduce the level of organic substances responsible for tastes and odors in water (4,11,45,59). Lin (42) reported that the use of chemical oxidation or aeration was usually included with activated carbon treatment. Problems may develop, however, when carbon particles passed through the filters (57) or when bacteria were sloughed from the filter due to hydraulic forces (69). Bacteria could readily colonize carbon filters (8,35,73) and become highly resistant to chlorination (35). LeChevallier et al. (35) studied carbon particles by scanning electron microscopy and found that bacteria grew in cracks and crevices and became covered with a slime layer. An attachment period of 20 minutes was sufficient to protect Escherichia coli cells from the effects of chlorine. The short attachment period did not allow for extracellular polymer production therefore indicating that attachment alone was adequate in protecting cells from the affects of chlorine.
Coliforms may be detected by either the most probable number (MPN) procedure or the membrane filter method (2). Many investigators have reported better coliform recovery with the MPN technique than the membrane filter method (6,7,32,46,60,62). These observations can be explained by the nonselective environment the MPN procedure provides allowing stressed or injured cells to repair themselves before coming in contact with selective media (6,7,67). As an example, Shipe and Cameron (62) compared the efficiency of the membrane filter and MPN techniques from three sources of water. Water from the Watauga River in Tennessee, Nashville tap water and double distilled water were sterilized and seeded with two concentrations of Escherichia coli. The high concentration was approximately 6-10 x 105 cells per ml and the low concentration was approximately 6-10 x 101 cells per ml. The results showed a MPN recovery of 108.9 per cent from the 3 water sources while the membrane filter method yielded recoveries of 47.6 percent. The average recoveries of both methods from the river water, tap water and distilled water were 47.8, 91.0 and 86.1 percent, respectively. The tap water and distilled water percentages indicated that either procedure produced adequate recovery. This lends support to the concept that the particular water sampled affects the efficiency of specific coliform enumeration procedures (16).
MATERIALS AND METHODS
Types of Bacteria Enumerated
Heterotrophic plate count bacteria were enumerated on Henrici
medium as modified by Stark and McCoy (66) and/or R2A agar (56) using
the spread plate technique or the membrane filter method (2), depending
upon the expected concentration of organisms. Actinomycetes were
detected using Starch Casein agar and the procedure outlined in Standard
Methods (2). Sulfate reducing bacteria were counted by the multiple
tube procedure of Mara and Williams (43), using their modification of
Iron Sulfite agar (Oxold). All of the above media were incubated at
20°C for 7 days except sulfate reducing bacteria which were incubated at
20°C for 30 days.
Clostridium perfringens were detected on Perfringens/O.P.S.P. medium (Oxoid) following the procedure outlined in the Oxoid Manual (54). Plates were incubated in an anaerobic jar for 24 h at 37°C. Semi-anaerobic conditions were obtained by the use of a GasPak (BBL Microbiology Systems, Cockeysville, Md.).
Fluorescent colonies were identified on Starch Casein agar plates by exposing plates to long-wave ultraviolet light.
Total coliform populations were determined by the 5-tube most probable number (MPN) technique using Lauryl Tryptose broth (Difco Laboratories, Detroit, MI.) in the presumptive test and Brilliant Green Bile broth (Difco) for confirmation (2).
Fecal coliforms and fecal streptococci were enumerated on Millipore HC membrane filters following the procedure outlined in Standard Methods (2). The media used were MFC (BBL) and KF (BBL), respectively. Fecal coliforms were also detected by transferring positive lauryl tryptose broth tubes to EC broth (BBL).
Water Distribution System
Drinking water to Laramie, Wyoming is supplied by ground water and
surface water sources. The ground water sources originate from one
spring and two well fields. The Laramie River serves as the surface
water source and is stored in a lake until use. A surface water
treatment plant processes water by flocculation/coagulation,
sedimentation, filtration, fluoridation, and chlorination. Ground water
receives fluoridation and chlorination only. Surface water is treated 20
miles west of Laramie and transported to town via gravity flow through a
20 and 24 inch main to the distribution system and storage reservoir.
Mixing of the three water sources is achieved in the distribution
system. One ground water source is treated within fifty yards of the
reservoir. The second ground water source is treated three miles south
of Laramie and can be sampled approximately 100 feet from the point of
treatment. Finished surface water can be sampled directly at the
treatment plant. The town's distribution system is supplied primarily
from the storage reservoir or the transmission lines.
Water Plant Study
Water samples from four points within the surface water treatment
plant were collected at weekly intervals from 14 May through 11 September, 1984.
The sampling sites included the following: raw water entering the plant, the
effluent from the sedimentation basin, filtered water and finished water.
Samples were collected aseptically in one-liter, sterile polypropylene bottles. Finished water samples were dechlorinated at the time of collection with 100 mg/L sodium thiosulfate. Samples were packed in ice, transported to the laboratory, and processed within 2 to 4 h of collection.
Organisms enumerated in this study included heterotrophic plate count bacteria, actinomycetes and sulfate reducing bacteria. Samples of raw water and the effluent from the sedimentation basin were tested at certain times for the presence of Clostridium perfringens to determine if this organism was a significant component of the sulfate reducing bacterial population.
Filter Study
The filters at the surface water treatment plant were studied to
determine the efficiency of filtration, and to see if any differences
existed between filters.
Six filters, at various hours after backwash, were sampled at daily intervals from July 17 to 19. Two filters were sampled a second time at 2, 26, 50 and 74 hours after backwash from August 22-25, 1984. Samples were handled as previously described. Heterotrophic plate count and sulfate reducing bacteria were enumerated in this study.
Sedimentation Basin Study
The sedimentation basin was divided into two parallel chambers,
each of which was 100 feet long. A walkway extended across the basin,
allowing access to the chambers. Ten sites, 10 feet apart, on
alternating sides of the walkway, were marked for sampling, with site
one being the closest to and site 10 being the most distant from the
mixing basin.
Samples of the sedimented material were obtained with the aid of a sampling device which consisted of electrical conduit attached end-to- end with compression couplings. A casting reel, filled with braided nylon line, was attached to one end of the device. Fishing rod guides of decreasing size were attached in equal intervals from the reel to about one foot from the tip. The line was threaded through the guides and attached to a number 10 rubber stopper to seal a 500 ml polypropylene bottle. A sterile bottle was attached to the tip section with tape, the cap removed, and the stopper inserted. The bottle was lowered into the basin until it disappeared into the sediment. The line was then pulled to remove the stopper, thus filling the bottle with the desired sample. The bottle was retrieved and cap returned. Samples were packed in ice, transported to the laboratory, and processed within 4 to 7 h of collection.
The sedimentation basin, which had not been cleaned since May, 1984, was sampled a total of 3 times from mid-August through mid-September, 1984. The final sampling was taken after a trial addition of powdered activated carbon (PAC), used in an attempt to remove a taste and odor from the water.
The organisms enumerated in this study included heterotrohic plate count bacteria, actinomycetes, sulfate reducing bacteria, fluorescent bacteria, total coliforms, fecal coliforms and fecal streptococci.
Survival of Escherichia coli in Sediment Samples
A fecal coliform, identified by means of an Enterotube II (Roche
Diagnostics, Division of Hoffman-LaRoche, Inc., Nutley, NJ) as
Escherichia coli. isolated from the Laramie Lagoon system, was
used for this and other parts of the study.
The survival of E. coli was studied in two sets of 4 sediment samples from the area of maximum deposition in the sedimentation basin. The second set of samples had received powdered activated carbon (PAC) in early September, 1984, in an endeavor to remove the aforementioned taste and odor.
Four sterile 8 oz prescription bottles and four Waring blender jars were used in this study. Each bottle was filled with a 100 ml aliquot of an unsterilized sample. The amount of sample was increased to 200 ml in blender jars. In order to cover the mixing blades with fluid. E. coli was grown in Nutrient Broth (Difco) at 37°C for 12 h. The bottles were inoculated with approximately 4.0 x 103 cells per ml whereas the blender jars received approximately 8.0 x 103 cells per ml. The containers were incubated at 15°C under stationary conditions and sampled at various intervals for approximately 3 mo. The prescription bottles were shaken 25 times and Waring jars blended 5 s on low speed prior to each plating.
The numbers of viable E. coli cells were determined by the membrane filtration procedure for fecal coliforms (2). Injured cells would not have been recovered by this methodology and hence would have been considered to be dead.
Polymer Effects on Escherichia coli
The Nalco 8102 polymer used in the coagulation process at the
surface water treatment plant was studied to determine if it affected
the growth of E. coli. The polymer was diluted to 0.22, 0.44,
0.66, 0.88 and 1.10 mg/L in Nephlo flasks (Belico Glass, Inc., Vineland, NJ.)
Each flask was inoculated with 0.1 ml of a 12-hour culture of E.
coli grown in trypticase soy broth at 37°C. The flasks were
incubated under stationary conditions. Growth was determined using a Klett-
Summerson Photoelectric Colorimeter fitted with a red (number 66) filter.
Distribution System Study
Samples from 6 sites thought to be representative of the various
parts of the distribution system were collected. These sites were
believed to include water supplied mainly from the following: 1) ground
water, 2) the surface water source and 3) that part of the system which
received a mixture of surface and ground water. Sites 1 to 4 were
private homes. Site 1 was located in the southern part of town, site 2
in the western, and sites 3 and 4 in the northern. Sites 5 and 6 were
from Soldier Springs and Pope well field treatment facility. The
sampling tap for this facility was located approximately 100 feet
post-treatment. Site 5 was the designation used for those samples that
were dechlorinated immediately while site 6 was the label for samples
from the same tap that were dechlorinated 1 h after collection. A
contact period of 1 h was thought to be a reasonable estimate of the
elapsed time before the treated water from this source could be
consumed.
Historically, sampling designs of distribution systems have been based upon samples from the line and not from samples obtained from the plumbing of the house (3). Sampling in this instance was designed to evaluate what microbes an individual might consume when obtaining a drink of water. Samples from the private homes were collected by individuals that worked in the Department of Microbiology and Veterinary Medicine at the University of Wyoming, Laramie, Wyoming. Instructions were to let the water run awhile before collecting the sample.
Heterotrophic bacterial plate counts and total coliforms were enumerated in this study.
Cup Sink Study
Five cup sinks in room 6035 of the Agriculture Building on the
campus of the University of Wyoming, Laramie, Wyoming, were studied to
determine the rate of repopulation by bacteria of a water line. The
addition to the building opened in August, 1982, hence a relatively new
water line was studied. The sinks had not been used for 22 days prior
to this study. In an effort to obtain a sample from the water line as
opposed to the spout, the first 100 ml of water from each tap was
discarded. The next 500 ml of flow was collected for analysis,
hereafter referred to as the before flush sample. After this collection
each tap was allowed to flow at full force for 30 minutes and then
sampled again. This will be referred to as the time zero sample. Flow
through the line was allowed only when samples were obtained at 8, 24
and 48 hours post-flushing.
Chlorine residual of these samples were determined using a Hach DPD Chlorine Test Kit (Hach Co., Loveland, Co.). If a chlorine residual was detected, the sample was dechlorinated with 100 mg/L of sterile sodium thiosulfate.
Identification of Organisms
Organisms were identified from the finished water at the surface
water treatment plant, the distribution system part of this study and
cup sink study. Colonies from Modified Henrici and R2A media were
purified on R2A agar plates and pure cultures were maintained on R2A
agar slants. All isolated cultures were placed into genera or groups
following the procedure outlined by LeChevallier et al. (36). Pigmented
bacterial isolates in this study which were otherwise identified as
Moraxella or Group M Moraxella-like organisms were called pigmented
Moraxella or pigmented Group M Moraxella-like organisms, respectively.
No further identification of these isolates was done.
Statistical Analysis
Statistical analysis of the data was made at the University of
Wyoming computer facility using the CYBER 835 computer. The statistical
tests performed included: Analysis of Variance with completely
randomized designs for 1 to 3 factors, Least-Squares Regression for data
with unequal subclass numbers, and Duncan's New Multiple Range Test
(33).
RESULTS
Surface Treatment Plant Study
The monthly averages of weekly samples taken at the surface water
treatment plant are presented in Table 1. Total heterotrophic bacteria
were enumerated using two methods; the spread plate procedure and the
membrane filter technique using modified Henrici medium and/or R2A
agar. When heterotrophic plate count bacteria were enumerated on
Modified Henrici agar the number of viable cells in the raw water was
significantly lower in August than any other month except June (P <
0.05). The highest number of cells leaving the sedimentation basin was
seen in September (P < 0.05). The highest number of viable bacteria,
in the finished water, 620 per 100 ml, was seen In June (P < 0.05).
These differences were not observed when this group of bacteria was
enumerated using R2A medium (P < 0.05). No significant differences in
the number of actinomycetes on Starch Casein agar plates were seen
between months within a sample site.
The numbers of sulfate reducing bacteria entering the plant each month were not significantly different; however, the highest number, 170 per 100 ml, passed the filter in June and the lowest number, 73 per 100 ml, were applied to the filter in August (P < 0.05). No sulfate reducing bacteria were detected in any sample after chlorination. To determine if these organisms were Clostridium perfringens, sixteen raw water and sedimentation basin effluent samples were plated on Perfringens agar with only one colony found. Hence, it was concluded that C. perfringens did not make-up a very large part of the population of the sulfate reducing bacteria enumerated in this study.
Table 1. AVERAGE MONTHLY PLATE COUNTS OF SELECTED BACTERIAL POPULATIONS AT VARIOUS SITES IN THE SURFACE WATER TREATMENT PLANT
| Site | Month | Heterotrophic Plate Counts Per 100 ML | |||||||||||||||
| Modified Henrici Medium | R2A Medium | Actinomycetes Per 100 ML | Sulfate Reducing Bacteria per 100 ML | ||||||||||||||
| Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples | ||||||
| RAW WATER | May | 1.4x106 | A## | 5.6x105 | 3 | 1.9x103 | A | 4.4x102 | 3 | ||||||||
| June | 6.7x105 | AB | 5.2x105 | 4 | 1.0x103 | A | 7.0x102 | 4 | 2.3x103 | A | 7.6x102 | 4 | |||||
| July | 1.5x106 | A | 6.1x105 | 4 | 1.1x106 | A | 0 | 1 | 1.6x103 | A | 9.4x102 | 4 | 2.4x103 | A | 1.3x103 | 4 | |
| Aug. | 4.5x105 | B | 2.5x105 | 5 | 7.4x105 | A | 3.8x105 | 5 | 9.2x102 | A | 1.8x102 | 5 | 2.3x103 | A | 3.0x103 | 5 | |
| Sept. | 1.6x106 | A | 9.7x105 | 2 | 5.7x105 | A | 2.0x105 | 2 | 8.5x102 | A | 2.1x102 | 2 | 7.8x102 | A | 5.9x102 | 2 | |
| EFFLUENT FROM SEDI- MENTATION BASIN | May | 2.6x105 | B | 1.2x104 | 3 | 4.2x102 | A | 2.6x102 | 3 | ||||||||
| June | 2.1x104 | C | 1.4x104 | 4 | 1.0x102 | A | 0 | 1 | 2.1x102 | AB | 8.3x101 | 4 | |||||
| July | 2.9x105 | B | 2.5x105 | 4 | 3.1x105 | A | 0 | 1 | 1.5x102 | A | 1.7x102 | 4 | 3.5x102 | A | 2.5x102 | 4 | |
| Aug | 1.3x105 | BC | 6.2x104 | 5 | 1.8x105 | A | 7.7x104 | 5 | 6.0x101 | A | 5.5x101 | 5 | 7.3x101 | B | 5.4x101 | 5 | |
| Sept. | 6.1x105 | A | 1.3x105 | 2 | 3.8x105 | A | 2.8x105 | 2 | * | A | 0 | 2 | 1.5x102 | AB | 7.1x101 | 2 | |
| EFFLUENT FROM FILTERS | May | 4.8x104 | A | 2.5x104 | 3 | 1.1x101 | B | 1.9x101 | 3 | ||||||||
| June | 3.1x104 | 3.4x104A | 3.4x104 | 4 | * | A | 0 | 1 | 1.7x102 | A | 1.5x102 | 4 | |||||
| July | 9.6x104 | A | 1.6x105 | 4 | 1.5x105 | A | 0 | 1 | * | A | 0 | 4 | 6.7x101 | AB | 4.7x101 | 4 | |
| Aug. | 2.7x105 | A | 2.7x105 | 5 | 1.1x105 | A | 7.4x104 | 5 | 2.0x101 | A | 4.5x101 | 5 | 6.6x100 | B | 1.5x101 | 5 | |
| Sept. | 2.4x105 | A | 3.0x105 | 2 | 1.1x105 | A | 2.5x104 | 2 | * | A | 0 | 2 | * | B | 0 | 2 | |
| FINISHED WATER | May | 3.3x102 | AB | 5.8x102 | 3 | * | A | 0 | 3 | ||||||||
| June | 6.2x102 | A | 2.5x102 | 4 | * | A | 0 | 1 | * | A | 0 | 4 | |||||
| July | 4.0x101 | B | 5.2x101 | 4 | 7.4x101 | A | 4.9x100 | 1 | 3.3x101 | A | 4.7x101 | 4 | * | A | 0 | 4 | |
| Aug. | 6.1x100 | B | 4.0x100 | 5 | 6.7x100 | A | 5.9x100 | 5 | * | A | 0 | 5 | * | A | 0 | 5 | |
| Sept. | 1.6x101 | B | 7.8x100 | 2 | 3.2x100 | A | 3.5x10-1 | 2 | * | A | 0 | 2 | * | A | 0 | 2 | |
When the plate counts on Modified Henrici agar were used to assess the efficiency of treatment it was found that coagulation and sedimentation removed an average of 76 percent of the incoming bacteria, filtration removed 47 percent of the organisms applied to the filters and chlorination killed 99.9 percent of the viable cells which passed the filters. Overall, 99.99 percent of the cells that entered the plant were either removed or killed. Furthermore, when analysis was made between sites, there were significant reductions in the number of actinomycetes and sulfate reducing bacteria due to coagulation and sedimentation (P < 0.05).
Filter Efficiency
The results of studying the efficiency of the filters are presented
in Table 2. Filters 1 and 2 were not in operation and had been covered
with finished water at the end of backwash. The effluent from both of
these filters, had less heterotrophic bacteria than operating filters
(P < 0.05). In the first study, (July 17 to 19), the number of
heterotrophic bacteria passing the filter, as determined with R2A
medium, was significantly higher as the run progressed while, in the
second experiment, there were no significant differences in numbers at
any time (P<0.05). In contrast, when the enumeration was made using
Modified Henrici agar, in only one of four cases with active filters
from the first study was there a significant increase in numbers of
bacteria with time of use and, in the second experiment, the counts
decreased with time of use (P<0.05).
TABLE 2. AVERAGE PLATE COUNTS OF SELECTED BACTERIAL POPULATIONS FROM FILTERS AT THE SURFACE WATER TREATMENT PLANT
| Heterotrophic Plate Counts Per 100 ML | ||||||||||||||
| Modified Henrici Medium | R2A Medium | Sulfate Reducing Bacteria Per ML# | ||||||||||||
| Filter | Hours of run after backwash | Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples | ||||
| JULY 17-19, 1984 | ||||||||||||||
| 0 | 5.0x100 | B# | 7.1x100 | 2 | 1.0x101 | B | 0 | 2 | * | A | 0 | 3 | ||
| 1 | 0 | 5.0x100 | B | 7.1x100 | 2 | 2.0x101 | B | 1.4x101 | 2 | * | A | 0 | 3 | |
| 0 | 1.0x102 | A | 2.1x101 | 2 | 1.0x102 | A | 7.1x100 | 2 | * | A | 0 | 3 | ||
| 0 | 8.0x101 | A | 1.1x102 | 2 | * | A | 0 | 2 | * | A | 0 | 3 | ||
| 2 | 0 | 5.0x100 | A | 7.1x100 | 2 | * | A | 0 | 2 | * | A | 0 | 3 | |
| 0 | 5.0x101 | A | 2.8x101 | 2 | 3.0x101 | B | 2.8x101 | 2 | * | A | 0 | 3 | ||
| 23 | 3.8x102 | A | 2.3x102 | 2 | 7.2x102 | B | 2.1x102 | 2 | 5.7x100 | A | 3.0x100 | 3 | ||
| 3 | 47 | 6.0x102 | A | 6.4x101 | 2 | 8.4x102 | B | 2.3x102 | 2 | 1.0x100 | B | 1.0x100 | 3 | |
| 71 | 9.8x102 | A | 3.1x102 | 2 | 1.7x103 | A | 0 | 2 | 2.3x100 | A | 1.5x100 | 3 | ||
| 23 | 5.5x102 | A | 2.1x102 | 2 | 4.4x102 | B | 8.5x101 | 2 | 8.5x100 | A | 3.5x100 | 3 | ||
| 4 | 47 | 7.0x102 | A | 1.6x102 | 2 | 7.8x102 | B | 2.8x101 | 2 | 3.0x100 | AB | 1.0x100 | 3 | |
| 71 | 1.2x103 | A | 7.2x102 | 2 | 1.5x103 | A | 2.1x102 | 2 | 2.7x100 | B | 1.2x100 | 3 | ||
| 27 | 4.3x102 | A | 9.9x101 | 2 | 5.8x102 | B | 2.8x101 | 2 | 6.7x10-1 | A | 5.8x10-1 | 3 | ||
| 5 | 51 | 1.2x102 | A | 0 | 2 | 7.3x102 | B | 5.6x101 | 2 | 1.0x100 | A | 1.7x100 | 3 | |
| 75 | 9.7x102 | A | 7.5x102 | 2 | 1.4x103 | A | 2.8x102 | 2 | 2.3x100 | A | 1.2x100 | 3 | ||
| 27 | 3.0x102 | B | 2.8x101 | 2 | 5.7x102 | B | 1.4x101 | 2 | 2.7x100 | A | 5.8x10-1 | 3 | ||
| 6 | 51 | 2.3x102 | B | 9.9x101 | 2 | 4.0x102 | B | 4.0x102 | 2 | 1.3x100 | A | 5.8x10-1 | 3 | |
| 75 | 1.9x103 | A | 4.2x102 | 2 | 1.9x103 | A | 2.1x102 | 2 | 2.7x100 | A | 1.5x100 | 3 | ||
| AUGUST 22-25, 1984 | ||||||||||||||
| 2 | 7.4x103 | B | 3.0x103 | 2 | 3.4x103 | A | 5.0x102 | 2 | 6.7x10-1 | A | 5.8x10-1 | 3 | ||
| 5 | 26 | 1.7x104 | A | 7.1x102 | 2 | 8.3x103 | A | 9.7x103 | 2 | * | A | 0 | 3 | |
| 50 | 1.8x103 | C | 6.4x102 | 2 | 1.8x103 | A | 3.5x102 | 2 | * | A | 0 | 3 | ||
| 74 | 2.5x103 | C | 0 | 2 | 1.5x103 | A | 1.1x103 | 2 | 3.3x10-1 | A | 5.8x10-1 | 3 | ||
| 2 | 1.4x104 | B | 2.8x103 | 2 | 2.2x103 | A | 9.2x102 | 2 | 3.3x10-1 | A | 5.8x10-1 | 3 | ||
| 6 | 26 | 2.0x104 | A | 1.4x103 | 2 | 3.1x103 | A | 5.0x102 | 2 | * | A | 0 | 3 | |
| 50 | 4.1x103 | C | 1.4x102 | 2 | 1.3x103 | A | 1.4x103 | 2 | * | A | 0 | 3 | ||
| 74 | 1.5x103 | C | 5.0x102 | 2 | 2.1x103 | A | 7.1x101 | 2 | * | A | 0 | 3 | ||
Sedimentation Basin Study
The averages of three samplings taken at the sedimentation basin
are given in Table 3. The bulk of the bacteria appeared to settle
within the first 30 linear feet of flow into the settling basins
(P<0.05). The evidence for this is that the highest numbers for the
heterotrophic plate count bacteria on Modified Henrici agar, the
actinomycetes, the fluorescent bacteria, the pigmented bacteria, the
fecal coliforms determined by the standard MF procedure and the fecal
streptococci were found within this distance. In contrast, the
heterotrophic plate count bacteria, as determined on R2A medium, and the
total coliforms and fecal coliforms determined by MPN procedures, which
can be considered resuscitation methods, showed no significant
differences in number throughout the settling basin (P<0.05). Sulfate
reducing bacteria appeared to settle equally throughout the sedimentation
basin. This observation could also be explained if these organisms were
actively growing in the sediment; however, no attempt was made to
determine this.
TABLE 3. AVERAGE PLATE COUNTS PER MILLILETER OF SELECTED BACTERIAL POPULATIONS IN SEDIMENT SAMPLES
| Sedimentation Basin Sample Number | Heterotrophic Plate Counts | |||||||
| Modified Henrici Medium | R2A Medium | Actinomycetes | Sulfate Reducing Bacteria | |||||
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |
| 1$ | 1.9x106@B | 1.5x106# | 2.5x106A | 1.0x106 | 2.4x105B | 2.2x105 | 3.2x104B | 4.2x104 |
| 2 | 2.1x106AB | 2.5x106 | 2.3x106AB | 1.2x106 | 2.2x105B | 2.1x105 | 6.8x104A | 1.1x105 |
| 3 | 2.5x106A | 2.7x106 | 1.4x106B | 8.4x105 | 3.9x105A | 5.2x105 | 2.8x104B | 2.9x104 |
| 4 | 1.7x106B | 1.0x106 | 2.3x106AB | 2.0x106 | 1.7x105C | 1.6x105 | 2.6x104B | 2.2x104 |
| 5 | 1.5x106B | 1.2x106 | 8.2x105B | 3.9x105 | 3.3x104E | 1.5x104 | 1.5x104B | 1.9x104 |
| 6 | 1.5x106B | 1.2x106 | 1.8x106AB | 1.2x106 | 1.2x105D | 1.6x105 | 3.7x104AB | 6.1x104 |
| 7 | 3.6x106C | 3.8x105 | 1.0x106B | 6.9x105 | 7.0x104DE | 4.4x104 | 1.4x104B | 9.5x103 |
| 8 | 6.3x105C | 7.4x105 | 1.3x106B | 9.0x105 | 5.2x104E | 5.9x104 | 1.3x104B | 1.5x104 |
| 9 | 3.3x105C | 9.6x104 | 1.4x106B | 1.5x105 | 3.1x104E | 2.6x104 | 9.3x103B | 7.8x103 |
| 10 | 6.0x105C | 3.7x105 | 1.6x106B | 4.4x105 | 4.6x104E | 4.7x104 | 6.4x103B | 6.5x103 |
| Fecal Coliforms Determined by | ||||||||
| Membrane Filtration | Most Probable Number | Fecal Streptococci | Total Coliforms | |||||
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |
| 1 | 1.1x101C | 1.7x101 | 3.2x101A | 4.1x101 | 1.5x101B | 1.0x101 | 6.3x101A | 6.0x101 |
| 2 | 8.5x101A | 1.4x102 | 3.4x102A | 5.0x102 | 4.3x101A | 6.6x101 | 4.0x102A | 4.5x102 |
| 3 | 4.0x101B | 5.4x101 | 8.2x101A | 6.7x101 | 4.0x101A | 6.1x101 | 7.8x101A | 5.6x101 |
| 4 | 6.7x100CD | 1.2x101 | 2.7x101A | 2.3x101 | 6.7x100B | 5.8x100 | 7.2x101A | 8.7x101 |
| 5 | 3.3x100CD | 5.8x100 | 2.6x101A | 8.6x100 | 6.7x100B | 2.9x100 | 1.5x102A | 1.7x102 |
| 6 | * | 0 | 4.0x101A | 4.6x101 | 8.3x100B | 7.6x100 | 2.3x102A | 2.4x102 |
| 7 | * | 0 | 5.9x100A | 4.4x100 | * | 0 B | 6.4x100A | 4.1x100 |
| 8 | 5.0x100CD | 5.0x100 | 1.2x101A | 4.6x100 | * | 0 B | 5.6x102A | 9.0x102 |
| 9 | * | 0 | 2.8x100A | 9.2x10-1 | 8.3x100B | 1.4x101 | 1.3x101A | 7.8x100 |
| 10 | * | 0 | 9.6x100A | 6.5x100 | 1.0x101B | 1.3x101 | 1.5x101A | 8.8x100 |
| Pigmented Bacteria | Flourescent Bacteria | |||||||
| Modified Henrici Medium | R2A Medium | |||||||
| Mean | Standard Deviation | Mean | Standard Dev iation | Mean | Standard Deviation | |||
| 1 | 2.5x105A | 3.1x105 | 1.9x105A | 1.7x105 | 8.7x104ABC | 4.9x104 | ||
| 2 | 1.7x105B | 2.2x105 | 1.4x105B | 2.0x105 | 1.3x105A | 5.9x104 | ||
| 3 | 2.0x105B | 2.6x105 | 1.2x105B | 1.8x105 | 8.3x104ABC | 9.4x104 | ||
| 4 | 1.2x105C | 1.7x105 | 6.0x104CD | 7.5x104 | 1.0x105AB | 7.0x104 | ||
| 5 | 4.7x104DE | 3.5x104 | 5.7x104CD | 6.0x104 | 3.0x100D | 5.2x103 | ||
| 6 | 8.7x104CD | 9.5x104 | 8.0x104C | 7.8x104 | 4.3x104BCD | 2.9x104 | ||
| 7 | 7.7x103E | 6.5x103 | 3.2x104D | 2.8x104 | 2.9x104C | 2.7x104 | ||
| 8 | 1.8x104E | 1.9x104 | 4.3x104D | 2.2x104 | 3.1x104C | 2.5x104 | ||
| 9 | 6.0x103E | 6.6x103 | 4.3x104D | 3.0x104 | 1.5x104D | 5.7x103 | ||
| 10 | 4.3x104DE | 5.3x104 | 6.5x140CD | 5.7x104 | 1.7x104D | 5.8x103 | ||
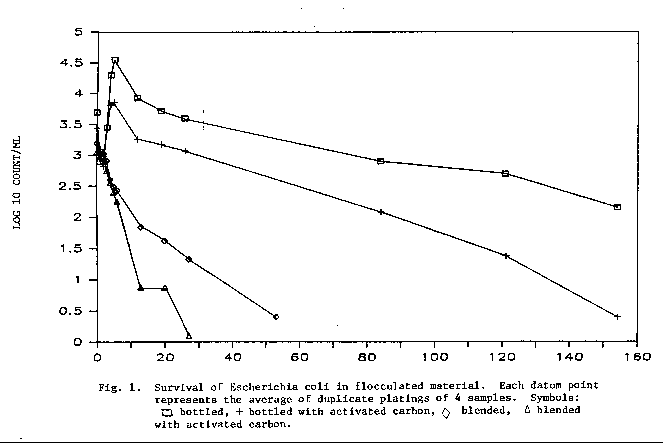
Escherichia coli Survival Study
The survival of E. coli inoculated into sedimented material is
presented in Fig. 1. The organism survived for a longer period of time
in the material that did not have powdered activated carbon (PAC) than
in the presence of PAC. The organism was also shown to survive longer
in material which was not blended than in the sediments which were
blended. Three percent of the cells remained viable in the unblended
material after 120 d. of storage at 15°C. This could mean that
bacterial cells, including those of pathogens, could survive for
extended time in flocculated material and, as Bulson et al. (9) stated,
the potential exists for disturbance and release of these organisms into
the water column. Survival could also be affected by the type of
sedimented material.
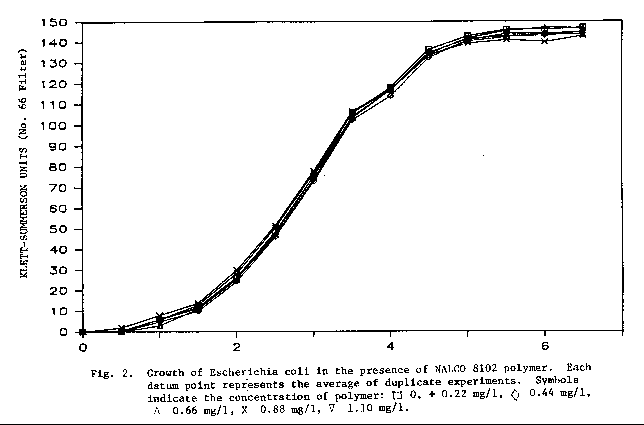
Polymer Effects on Escherichia Coli
The surface water treatment plant uses Nalco 8102 polymer to
produce a water insoluble floe. The results of this study are shown in
Fig. 2. In the laboratory, E. coli was grown in polymer
concentrations that may reasonably be encountered within the treatment process.
No observed effect was seen during the first 6 hours of growth between the
control culture and those with polymer. Monitoring the growth of E.
coli for up to 30 hours also yielded no observable effects.
Distribution System Study
Data from the distribution system study are given in Table 4. The
heterotrophic plate counts in water from three homes were significantly
higher than counts from water at the other home and the two differently
dechlorinated ground water samples (P<0.01). Furthermore, two of the
three homes had significantly higher numbers of bacteria in the drinking
water during the fall of the year than in December (P<0.01). The
heterotrophic plate count at one home was significantly higher from R2A
medium than when Modified Henrici agar was used (P<0.01). Counts from
the remaining samples on R2A medium, although not statistically
significant, were equal to or higher than those on Modified Henrici
agar. There was no apparent difference in numbers of bacteria in any
particular part of town. Total coliforms were not detected from any
sampling site.
TABLE 4. AVERAGE HETEROTROPHIC PLATE COUNTS PER 100 ML FROM THE DISTRIBUTION SYSTEM.
| Modified Henrici Medium | R2A Medium | |||||
| Site | Mean | Standard Deviation | Number of Samples | Mean | Standard Deviation | Number of Samples |
| September | ||||||
| 1 | 1.5x103A## | 1.4x103 | 5 | 2.4x103A | 1.1x103 | 5 |
| 2 | 7.3x102B | 7.7x102 | 6 | 7.7x102B | 6.8x102 | 6 |
| 3 | 1.0x103AB | 9.5x102 | 3 | 1.0x103B | 9.0x102 | 3 |
| 4 | 2.1x102C | 3.3x102 | 6 | 2.0x102C | 3.3x102 | 6 |
| 5 | 3.8x101C | 5.4x101 | 6 | 3.9x101C | 5.0x101 | 6 |
| 6 | 7.8x103C | 1.2x101 | 6 | 8.7x100C | 9.8x100 | 6 |
| December | ||||||
| 1 | 3.7x102AB | 2.7x102 | 5 | 4.1x102AB | 3.3x102 | 5 |
| 2 | 6.8x102A | 1.2x102 | 5 | 7.4x102A | 1.4x102 | 5 |
| 3 | 2.6x102AB | 1.8x102 | 4 | 2.9x102AB | 2.0x102 | 4 |
| 4 | 7.3x101B | 1.2x101 | 5 | 9.7x101B | 1.6x102 | 5 |
| 5 | 2.2x101B | 1.2x101 | 3 | 2.2x101B | 1.0x101 | 3 |
| 6 | 2.7x100B | 1.2x100 | 3 | 3.0x100B | 2.0x100 | 3 |
Cup Sink Study
Data concerned with the repopulation of five cup sink taps in room
6035 of the Agriculture Building are presented in Table 5. There was a
significant difference in the results from the two experiments (P<0.01).
The first time taps 4 and 5 were flushed the heterotrophic plate counts
dropped from 120 to 75 and 720 to 420 cells per ml, respectively from
the start until the end of flushing, and there was little change in
numbers for the next 48 h. In contrast, there was at least a 90 percent
reduction in numbers in the other taps and in all taps during the repeat
experiment. The reason or this is unclear; however, taps 4 and 5 were
rarely used before the first experiment. Generally, the flushing of the
taps reduced the bacterial numbers by 90 to 99 percent, and there was
gradual repopulation of the taps as the chlorine residual was depleted.
TABLE 5. AVERAGE HETEROTROPHIC PLATE COUNTS OF SAMPLES FROM CUP SINKS
| Time | Chlorine Residual | Sink Tap Number | |||||||||
| 1 | 2 | 3 | 4 | 5 | |||||||
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| 27 November | |||||||||||
| Before Flush | ND | 8.8x103@A | 2.0x102## | 8.0x103A | 9.2x101 | 1.5x103A | 3.5x102 | 1.2x102A | 9.2x101 | 7.2x102A | 2.1x101 |
| Time Zero | 0.1 | 4.5x101C | 3.5x101 | 6.0x101D | 1.4x101 | 1.2x102B | 2.1x101 | 7.5x101A | 5.0x101 | 4.2x102B | 1.9x102 |
| 8h | 0 | 1.2x102C | 7.1x100 | 2.9x102C | 1.4x101 | 1.6x102B | 7.8x101 | 1.0x102A | 5.6x101 | 1.0x102C | 2.1x101 |
| 24h | ND | 1.3x102C | 1.4x101 | 4.1x102C | 3.5x101 | 3.2x102B | 2.1x101 | 1.2x102A | 2.1x101 | 8.0x101C | 2.8x101 |
| 48h | ND | 4.6x102B | 1.4x102 | 1.9x103B | 7.1x100 | 2.5x102B | 1.4x101 | 5.0x101A | 1.4x101 | 3.0x101C | 1.4x101 |
| 12 December | |||||||||||
| Before Flush | ND | 3.8x103 | 2.3x103A | 6.4x103 | 2.5x102 | 4.4x103A | 4.0x102 | 4.9x103A | 4.2x102 | 5.6x103A | 3.2x102 |
| Time Zero | 0.2 | 6.3x100C | 1.4x10-1 | 1.3x101C | 1.1x100 | 1.9x101C | 2.8x100 | 1.4x101 | 1.1x100 | 2.9x101 | 2.3x100 |
| 8h | 0 | 6.5x101C | 7.1x100 | 1.0x102C | 2.8x101 | 1.7x102C | 1.4x101 | 1.1x102 | 4.2x101 | 7.0x101 | 1.4x101 |
| 24h | 0 | 2.3x102C | 1.8x102 | 1.7x102C | 4.2x101 | 3.4x102C | 5.6x101 | 4.0x102 | 6.4x101 | 3.9x102 | 1.4x101 |
| 48h | ND | 6.7x102B | 9.9x102 | 9.6x102B | 5.0x101 | 1.5x103B | 7.1x100 | 7.8x102 | 2.1x101 | 1.8x103 | 3.8x102 |
Bacterial Identified from Finished Water
The identification of the bacteria found in finished water are
given in Table 6. All isolated cultures were placed into genera or
groups following the procedure outlined by LeChevallier et al. (36).
There did not appear to be any specific organism at any specific
location in the distribution system; therefore all sites were lumped
together. The two predominant bacteria were identified as nonpigmented
as Group M Moraxella-like, which comprised 22 percent of the isolates,
and pigmented Moraxella species which were 26 per cent of the total.
The most frequently isolated bacterium from the finished surface water
at the treatment plant was identified as pigmented Group M
Moraxella-like bacteria whereas Micrococcus roseus/M.
varians and actinomycetes were each isolated 23 percent of the time from
the finished ground water. Based upon the limited number of isolates in
this study there did not appear to be a seasonal effect. Members of the
Acinetobacter, Corynebacterium, nonpigmented Moraxella and
the actinomycete group were not found in the finished surface water but were
isolated from finished ground water.
TABLE 6. IDENTIFICATION OF HETEROTROPHIC PLATE COUNT BACTERIA
| Organism | Finished Surface Water | Finished Ground Water | Distribution Water | |||
| Total | % of Total | Total | % of Total | Total | % of Total | |
| Acinetobacter spp. | 0 | 0 | 2 | 6.7 | 9 | 7.3 |
| Actinomycete | 0 | 0 | 7 | 23.3 | 2 | 1.6 |
| Alcaligenes spp. | 1 | 3.1 | 0 | 0 | 0 | 0 |
| Arthrobacter spp. | 5 | 15.6 | 1 | 3.3 | 11 | 8.9 |
| Bacillus spp. | 1 | 3.1 | 4 | 13.3 | 5 | 4.0 |
| Corynebacterium spp. | 0 | 0 | 1 | 3.3 | 0 | 0 |
| Group M (nonpigmented) | 1 | 3.1 | 2 | 6.7 | 27 | 21.8 |
| Group M (pigmented) | 9 | 28.1 | 3 | 10.0 | 13 | 10.5 |
| Micrococcus luteus | 4 | 12.5 | 1 | 3.3 | 1 | 0.8 |
| Micrococcus roseus/M. varians | 4 | 12.5 | 7 | 23.3 | 9 | 7.3 |
| Moraxella sp. (nonpigmented) | 0 | 0 | 1 | 3.3 | 12 | 9.7 |
| Moraxella sp. (pigmented) | 2 | 6.2 | 0 | 0 | 32 | 25.8 |
| Unidentified | 5 | 15.6 | 1 | 3.3 | 3 | 2.4 |
| Total | 32 | 99.8 | 30 | 99.8 | 124 | 100.1 |
DISCUSSION
One objective of this study was to determine if the presence and growth of actinomycetes were responsible for the tastes and odors that developed in Laramie's drinking water during the warm summer months. Surface water was identified as the potential source of the problem because tastes and odors quickly subsided when the surface water treatment plant closed for the season in September. The number of actinomycetes detected from water in various stages of treatment was not significantly different before or after the appearance of the taste and odor problem. Although this does not prove that actinomycetes were not partly responsible for the aesthetic problem. It does indicate that investigations should be expanded to include other groups of aquatic organisms, such as algae, to adequately identify the causative agent.
The number of heterotrophic bacteria in the raw water at the surface water treatment plant remained reasonably stable except during the August enumeration with Modified Henrici agar. No significant decrease in counts was detected with R2A medium during this month. The reason for this is unclear.
In general, the heterotrophic plate counts from the effluent of the sedimentation basin as determined by Modified Henrici agar changed significantly with each month of sampling while counts on R2A medium showed no difference. R2A medium enumerated a higher percentage of the heterotrophic bacterial population in July and August than did Modified Henrici agar; however, counts during September indicated that Modified Henrici medium was superior. The treatment plant closed for the season in mid-September and a limited number of samples were collected. Perhaps the trend noticed in July and August would have been seen during September if more samples had been available.
When comparisons were made by lumping the counts from the effluent of active and inactive filters it was found that Modified Henrici agar enumerated a significantly greater portion of the heterotrophic bacterial population than did R2A medium; however, when only active filters were analyzed R2A medium produced significantly higher counts. Cell injury during filters operation could explain this observation (8). During the filtering process cells trapped in the filter may become injured, and as filter study 1 indicated, the number of organisms passing the filter increased with time of operation. This suggests that a portion of the injured cells are released to the water column. The carbon and energy sources in R2A medium may more closely meet the nutritional requirements of these stressed cells.
Significantly higher numbers of actinomycetes, fluorescent bacteria, pigmented bacteria and fecal streptococci were seen to settle within the first 30 linear feet of flow into the sedimentation basin. This may suggest that bacteria did not settle equally within the basin but settled in specific locations i.e. near the influent of the basin. Heterotrophic bacteria enumerated with Modified Henrici medium and fecal coliforms detected with the standard MF procedure additionally showed this trend of early settling. Enumeration of total and fecal coliforms with resuscitation procedures, and heterotrophic bacteria with R2A medium indicated that settling in the basin was equally distributed. Resuscitation techniques have often been reported to detect higher numbers of physiologically injured total and fecal coliforms than the standard MF procedure (7,62,67). Flocculated material has previously been shown to cause injury to Escherichia coli (9). This suggests that other groups of bacteria could also be susceptible to injury from the flocculated material. Data from the present study indicates that R2A medium may recover a higher percentage of the injured heterotrophic bacterial population from flocculated material than Modified Henrici agar.
E. coli was found to remain viable for up to 5 months at 15°C in flocculated material from the sedimentation basin. The addition of powdered activated carbon (PAC) appeared to accelerate the die-off of this organism. The literature indicated that the surface of activated carbon particles provide an ideal habitat for bacterial colonization (4,8,11,73). LeChevallier et al. (35) have noticed that bacteria accumulate in cracks and crevices on the carbon particle, become covered with a slime layer, and are well protected from the effects of chlorine. E. coli in the present study could be expected to attach to the PAC surface in the same manner, remain undetected and give the impression that rapid inactivation is occurring. Investigations by scanning electron microscopy would reveal if colonization was actually taking place.
In addition, E. coli seemed to be inactivated at a faster rate when samples were blended for 5 s. as opposed to shaken. In a similar study, Bulson et al. (9) found that blending for up to 18 seconds did not result in significant injury to E. coli. Other than blending, the only difference between the two samples was the nature of the container each was incubated in. Shaken samples were held in glass bottles while blended samples were in stainless steel containers. Studies with glass blender jars would be useful in determining if blending, the type of container, or both were responsible for the higher die-off of E. coli.
The coagulation and flocculation techniques practiced at the surface water treatment plant are much like those used to remove bacteria from the water column of lakes. Bulson et al. (9) recommended that flocculation of lakes for the removal of bacteria be performed at times when recreational use had subsided for the season to reduce the chance of individuals coming in contact with the floc. The survival of indicator bacteria and pathogens within the flocculated material from sedimentation basins may closely approximate the survival rates of bacteria removed from the water column of lakes by the same method. Sedimentation basin survival studies may prove useful in predicting the die-off of pathogens in the flocculated material and the potential health hazard before the actual treatment of lakes is initiated.
Past studies have linked sulfate reducing bacteria with accelerated corrosion and tubercle formation on the interior of pipelines (10,37,53,70). Deterioration of distribution systems in this manner can greatly affect the maintenance costs and longevity of water mains. Tuovinen et al. (70) indicated that when preventive measures are not practiced, extensive distribution system breakdown can result. The number of sulfate reducing bacteria entering the surface water treatment plant during May through September were considerable; however no bacteria from this group were ever detected in this finished water leaving the plant. Sulfate reducing bacteria may grow once in the distribution system, and monitoring this group of organisms should be continued to determine if the distribution network is susceptible to tuberculation.
The classification scheme outlined by LeChevallier et al. (36) allowed quick and easy identification of waterborne bacteria to the genus or group level. None of the species of Moraxella produce any pigment (38). For this reason a distinction was made between pigmented and nonpigmented isolates identified as Moraxella species or Group M Moraxella-like organisms. These organisms were suspected of belonging to the Pseudomonas and Flavobacterium genera, respectively. Further biochemical tests would have had to have been included to adequately identify these pigmented organisms if time had allowed. These tests would have included the following: oxidation of maltose and lactose, hydrogen sulfide production, arginine dihydrolase synthesis, and the absence of lysine decarboxylase for suspected Pseudomonas isolates, and ONPG for b- galactosidase production, oxidase synthesis, and the oxidation of mannitol for isolates thought to be Flavobacterium (38,61).
Heterotrophic plate count bacteria in drinking water may establish themselves in niches within the distribution system (1,53,70). The predominant organisms identified from the surface water treatment plant (pigmented Group M Moraxella-like) and ground water sources (actinomycetes and Micrococcus roseus/M. varians) differed from the predominant organisms found in distribution water (pigmented Moraxella sp. and nonpigmented Group M Moraxella-like). This may indicate that the environment of the distribution system is selective for certain types of bacteria.
LeChevallier et al. (36) used the same identification scheme for bacteria isolated from raw and finished drinking water supplied to an Oregon coastal town. The predominant strains identified from the raw water source included Acinetobacter spp., Aeromonas spp. and Enterobacter agglomerans, actinomycetes, Corynebacterium spp. and Aeromonas spp. constituted the largest portion of organisms from distribution water. This could be an indication of the bacterial habitats within the distribution system selecting for certain types of organisms (17).
Data from the distribution study show that great variations can exist between the heterotrophic plate counts from private homes. The differences that were seen between sampling sites might well be due to differences in how various people took the samples since the home with the lowest number of bacteria and the treatment facility were sampled by the same person.
LITERATURE CITED
APPENDIX I
Media Formulations
Modified Iron Sulfite Agar (Oxoid)
| Iron Sulfite Agar (Oxoid) | 23.0 g |
| FeSO4 * 7H2O | 0.5 g |
| Sodium Lactate 60% | 5.8ml |
| MgSO4 * 7H2O | 2.0 g |
| Distilled water | 1.0 l |
Supplement
| Ascorbic Acid | 7.5 g |
| Sodium Thioglycollate | 7.5 g |
| Distilled water | 100.0ml |
Add 0.5 ml of supplement to each 50 ml of melted medium. Dispense 10 ml of medium into inoculated culture tubes. Mix well. Allow to solidify. Add 3-4 ml of agar water for overlay.
Modified Henrici's Medium
| Peptone | 0.5 g |
| Glycerine | 0.5ml |
| Soluble Starch | 0.5 g |
| Sodium Caseinate | 0.5 g |
| K2HPO4 | 0.3 g |
| MgSO4 * 7H2O | trace |
| FeSO4 * 7H2O | 15.0 g |
| Distilled water | 1.0l |
R2A Medium
| Yeast Extract | 0.5 g |
| Proteose Peptone no. 3 (difco) | 0.5 g |
| Casamino Acids | 0.5 g |
| Glucose | 0.5 g |
| Soluble Starch | 0.5 g |
| Sodium Pyruvate | 0.3 g |
| K2HPO4 | 0.3 g |
| MgSO4 * 7H2O | 0.05 g |
| Agar | 15.0 g |
| Distilled | 1.0 l |
Starch-Casein Agar
| Soluble Starch | 10.0 g |
| Casein | 0.3 g |
| KNO3 | 2.0 g |
| NaCl | 2.0 g |
| K2HPO4 | 2.0 g |
| MgSO4 * 7H2O | 0.05 g |
| CaCO3 | 0.02 g |
| FeSO4 * 7H2O | 0.01 g |
| Agar | 15.0 g |
| Distilled water | 1.0 l |
To use:
Melt 50 ml amounts and dispense about 15.0 ml to sterile petri
plates. Allow to solidify. Melt 17.0 ml amounts and add 1 ml of
sterile Cycloheximide* (1 mg/ml) at the time of inoculation. Add 2 ml
of the appropriate dilution. Mix. Layer the previously poured plates
with 5 ml of this mixture. Incubate at 20°C for 7 days.
*Actldione, Upjohn Co. or equivalent
Legend for Appendix II
| + = positive reaction or utilization | |
| - = negative reaction or no utilization | |
| N = not done | |
| Morphology | 1 rod |
| 2 cocci or coccobacillus | |
| 3 branching filamentous hyphae | |
| Pigment | 1 colorless, white to cream |
| 2 yellow | |
| 3 red, orange | |
| Glucose fermentation | 1 no change |
| Flagella | 1 peritrichous |
| Cell Morphology | 1 rod to coccus transformation |
| 2 snapping division | |
APPENDIX II
Gram OF
Reaction Morphology Glucose Pigment Oxidase Indole Flagella
------------------------------------------------------------------------------------------------
Actinomycete + 3 M N N N N
Arthrobacter spp. + 1 M N N N N
Bacillus spp. + 1 M N N N N
Corynebacterium spp. + 1 M N N N N
Micrococcus Luteus + 2 1 2 N N N
Microccus roesus/varians + 2 1 3 N N N
Acinetobacter spp. - 2 1 N - N N
Alcaligenes spp. - 1 1 N N N 1
Group M (nonpigmented) - 1 1 1 N - N
Group M (pigmented) - 1 1 3 N - N
Moraxella sp. (nonpigmented) - 1 1 1 + N N
Moraxella sp. (pigmented) - 1 1 2 + N N
Cell
Morphology Motility Endospores
----------------------------------------------------------------------------------------------
Actinomycete N N N
Arthrobacter spp. 1 N -
Bacillus spp. N N +
Corynebacterium spp. 2 N -
Micrococcus Luteus N N N
Microccus roesus/varians N N N
Acinetobacter spp. N + N
Alcaligenes spp. N - N
Group M (nonpigmented) N - N
Group M (pigmented) N N N
Moraxella sp. (nonpigmented) N N N
Moraxella sp. (pigmented) N N
APPENDIX III
Average Weekly Plate Counts of Selected Bacterial Populations at Various Sites in the Surface Water Treatment Plant
| Site | Date | Heterotrophic Plate Counts per 100 ml | Actinomycetes per 100 ml |
Sulfate # Reducing Bacteria per 100 ml | |
| Modified Henrici agar | R2A agar | ||||
| Raw Water | 14 May | 7.8x105##@ | 1.4x103 | ||
| 22 May | 1.7x106 | 2.1x103 | |||
| 31 May | 1.8x106 | 2.2x103 | |||
| 6 June | 1.0x106 | 2.0x102 | 3.3x103 | ||
| 13 June | 3.8x105 | 1.8x103 | 1.5x103 | ||
| 20 June | 8.2x104 | 8.0x102 | 2.0x103 | ||
| 26 June | 1.2x106 | 1.4x103 | 2.4x103 | ||
| 5 July | 2.0x106 | 2.2x103 | 1.4x103 | ||
| 12 July | 5.9x105 | 8.0x102 | 1.1x103 | ||
| 19 July | 1.7x106 | 8.0x102 | 3.3x103 | ||
| 25 July | 1.6x106 | 1.1x106 | 2.6x103 | 3.6x103 | |
| 6 August | 7.6x105 | 6.8x105 | 1.0x103 | 7.6x103 | |
| 9 August | 4.0x105 | 1.3x106 | 8.0x102 | 1.0x103 | |
| 14 August | 3.9x105 | 3.4x105 | 8.0x102 | 1.2x103 | |
| 21 August | 6.0x105 | 9.8x105 | 1.2x103 | 1.0x103 | |
| 30 August | 9.8x104 | 4.7x105 | 8.0x102 | 6.6x102 | |
| 5 September | 2.3x106 | 4.3x105 | 1.0x103 | 1.2x103 | |
| 11 September | 9.3x105 | 7.1x105 | 7.0x102 | 3.6x102 | |
| Effluent from Sedimentation Basin | 14 May | 2.8x105 | 1.0x102 | ||
| 22 May | 2.6x105 | 5.7x102 | |||
| 31 May | 2.6x105 | 5.3x102 | |||
| 6 June | 1.6x104 | * | 1.0x102 | ||
| 13 June | 4.5x103 | * | 3.0x102 | ||
| 20 June | 2.7x104 | * | 2.3x102 | ||
| 26 June | 3.8x104 | 1.0x102 | 2.0x102 | ||
| 5 July | 1.4x105 | 1.0x102 | 1.7x102 | ||
| 12 July | 6.4x104 | * | 6.7x102 | ||
| 19 July | 3.4x105 | 1.0x102 | 1.3x102 | ||
| 25 July | 6.2x105 | 3.1x105 | 4.0x102 | 4.3x102 | |
| 6 August | 1.4x105 | 1.5x105 | * | 1.3x102 | |
| 9 August | 1.1x105 | 3.0x105 | 1.0x102 | 1.0x102 | |
| 14 August | 2.2x105 | 1.0x105 | 1.0x102 | * | |
| 21 August | 1.2x105 | 2.1x105 | 1.0x102 | 3.3x101 | |
| 30 August | 4.8x104 | 1.5x105 | * | 1.0x102 | |
| 5 September | 7.1x105 | 5.7x105 | * | 2.0x102 | |
| 11 September | 5.2x105 | 1.8x105 | * | 1.0x102 | |
| Effluent from Filters | 14 May | 7.3x104 | * | ||
| 22 May | 4.7x104 | 3.3x101 | |||
| 31 May | 2.3x104 | * | |||
| 6 June | 8.1x104 | * | 3.7x102 | ||
| 13 June | 4.5x103 | * | 1.3x102 | ||
| 20 June | 2.0x104 | * | * | ||
| 26 June | 1.8x104 | * | 1.7x102 | ||
| 5 July | 2.8x104 | * | 1.0x102 | ||
| 12 July | 5.0x103 | * | 1.0x102 | ||
| 19 July | 5.0x103 | * | * | ||
| 25 July | 3.4x105 | 1.5x105 | * | 6.7x101 | |
| 6 August | 7.3x105 | 1.9x105 | * | * | |
| 9 August | 2.0x105 | 1.9x105 | 1.0x102 | * | |
| 14 August | 2.0x105 | 4.8x104 | * | * | |
| 21 August | 2.0x105 | 6.8x104 | * | * | |
| 30 August | 2.1x104 | 5.1x104 | * | 3.3x101 | |
| 5 September | 2.6x104 | 1.3x105 | * | * | |
| 11 September | 4.5x105 | 9.4x104 | * | * | |
| Finished Water | 14 May | * | * | ||
| 22 May | 1.0x103 | * | |||
| 31 May | * | * | |||
| 6 June | 5.0x102 | * | * | ||
| 13 June | 1.0x103 | 1.0x102 | * | ||
| 20 June | 5.0x102 | * | * | ||
| 26 June | 5.0x10-1 | 3.2x101 | * | ||
| 5 July | 5.0x10 | * | * | ||
| 12 July | 2.0x100 | * | * | ||
| 19 July | 1.1x102 | 7.8x101 | * | * | |
| 25 July | 4.8x101 | 7.1x101 | * | * | |
| 6 August | 2.0x100 | 4.5x100 | * | * | |
| 9 August | 1.2x101 | 1.6x101 | * | * | |
| 14 August | 1.0x101 | 9.0x100 | * | * | |
| 21 August | 3.0x100 | 2.0x100 | * | * | |
| 30 August | 4.0x100 | 2.0x100 | * | * | |
| 5 September | 2.2x101 | 3.0x100 | * | * | |
| 11 September | 1.1x101 | 3.5x100 | * | * | |
Water Resources Publications List
Water Resources Data System Library |
Water Resources Data System Homepage