Wyoming Water Resources Center

|
RESEARCH BRIEFS Wyoming Water Resources Center |

|
Investigators: K.J. Reddy, Wyoming Water Resources Center, University of Wyoming; Z. Zhang, Wyoming Water Resources Center, University of Wyoming
Purpose: The objective of this study was to examine the feasibility of a chemical reduction process to remove nitrate from ground water. Nitrate is a commonly found contaminant of ground water, due to a variety of agricultural, industrial, and domestic practices. Excessive nitrate in drinking water can cause methemoglobinemia in humans; the current drinking water standard for nitrate (N-NO33-) is 10 mg/l. We initiated the research because future ground water remediation projects may require simple and economical processes to remove nitrate, particularly where alternate water supplies are not available. Current remediation techniques (e.g., biological denitrification, anion exchange, reverse osmosis) are time consuming and produce by-products, which require additional treatments.
 Methods: Recently, in the Wyoming Water Resources Center's
Environmental and Water Quality laboratory, we designed and developed a
redox controlling device (RCD) to determine redox potential effects on the
concentration of dissolved selenium, arsenic, and chromium. We have applied
RCD to examine the chemical reduction process of nitrate in ground water.
RCD consisted of a sampler chamber with pH, platinum (Pt), and reference
electrodes which were connected to the pH/Eh meter. The pH/Eh meter was
connected to a relay, O2, and H2 gas tanks to maintain desired redox
potential (Eh) in the sample. The O2 gas tank consisted of 95% O2 and 5%
CO2. The H2 gas tank consisted of 3% H2, 5% CO2, and 92% Argon. The
purpose of CO2 was to maintain a constant pH (5.0 ± 0.1) in the sample. A
peristaltic pump was used to circulate ground water in the sample chamber.
The water samples used in this study were collected from a reclaimed
surface coal mine site in the Powder River Basin, Wyoming. Two hundred mL
of ground water samples were diluted with 1000 mL of distilled-deionized
water. These samples were amended with 0.5g of carbon black catalyst
containing 10% platinum and were reacted at a given redox potential for
three different time periods: 24 hours, 1 hour, and 30 minutes. Samples
were collected from the sample port with a syringe and were analyzed
immediately for nitrate and nitrite (NO2-) by ion chromatography using
manual injection through a 0.45-µ M filter.
Methods: Recently, in the Wyoming Water Resources Center's
Environmental and Water Quality laboratory, we designed and developed a
redox controlling device (RCD) to determine redox potential effects on the
concentration of dissolved selenium, arsenic, and chromium. We have applied
RCD to examine the chemical reduction process of nitrate in ground water.
RCD consisted of a sampler chamber with pH, platinum (Pt), and reference
electrodes which were connected to the pH/Eh meter. The pH/Eh meter was
connected to a relay, O2, and H2 gas tanks to maintain desired redox
potential (Eh) in the sample. The O2 gas tank consisted of 95% O2 and 5%
CO2. The H2 gas tank consisted of 3% H2, 5% CO2, and 92% Argon. The
purpose of CO2 was to maintain a constant pH (5.0 ± 0.1) in the sample. A
peristaltic pump was used to circulate ground water in the sample chamber.
The water samples used in this study were collected from a reclaimed
surface coal mine site in the Powder River Basin, Wyoming. Two hundred mL
of ground water samples were diluted with 1000 mL of distilled-deionized
water. These samples were amended with 0.5g of carbon black catalyst
containing 10% platinum and were reacted at a given redox potential for
three different time periods: 24 hours, 1 hour, and 30 minutes. Samples
were collected from the sample port with a syringe and were analyzed
immediately for nitrate and nitrite (NO2-) by ion chromatography using
manual injection through a 0.45-µ M filter.
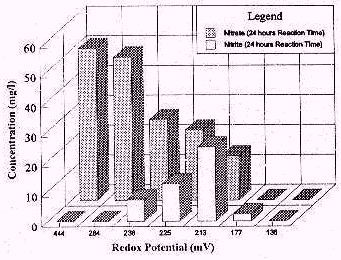
 Results: As the redox potential decreased, nitrate concentration in
ground water also decreased. A 33% reduction in nitrate concentration was
observed at a redox potential of 236 millivolts (mV). At a redox potential
of 177 mV, a 100% reduction in nitrate concentration was observed. A
synchronous increase in the concentration of nitrite was noticed with a
decrease in the concentration of nitrate. However, nitrite concentrations
began to decrease and were diminished by 100% at redox potential of 136 mV.
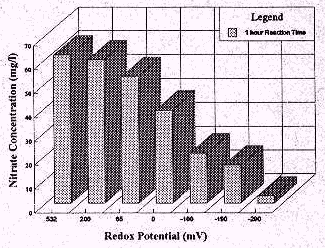
When the reaction time is reduced from 24 hours to 1 hour, 77% reduction
in nitrate concentration was observed at -100 mV. At a redox potential of
-290, a 93% reduction in the concentration of nitrate was noticed. When
the reaction time was further reduced to 30 minutes, the chemical reduction
process removed only 28% of nitrate at redox potential of -100 mV. These
results emphasize that shorter reaction times require lower redox potential
to remove nitrate from ground water. When the redox potential was increased
back to 603 mV, very little or no nitrate or nitrite was recovered. This
suggests that nitrate and nitrite were probably converted to nitrous and/or
nitric oxide.
Results: As the redox potential decreased, nitrate concentration in
ground water also decreased. A 33% reduction in nitrate concentration was
observed at a redox potential of 236 millivolts (mV). At a redox potential
of 177 mV, a 100% reduction in nitrate concentration was observed. A
synchronous increase in the concentration of nitrite was noticed with a
decrease in the concentration of nitrate. However, nitrite concentrations
began to decrease and were diminished by 100% at redox potential of 136 mV.
When the reaction time is reduced from 24 hours to 1 hour, 77% reduction
in nitrate concentration was observed at -100 mV. At a redox potential of
-290, a 93% reduction in the concentration of nitrate was noticed. When
the reaction time was further reduced to 30 minutes, the chemical reduction
process removed only 28% of nitrate at redox potential of -100 mV. These
results emphasize that shorter reaction times require lower redox potential
to remove nitrate from ground water. When the redox potential was increased
back to 603 mV, very little or no nitrate or nitrite was recovered. This
suggests that nitrate and nitrite were probably converted to nitrous and/or
nitric oxide.
Future Research: Future research will examine efficiency of other catalysts and possibility of testing chemical reduction process under field conditions.
Publications:
Gadlin, P.T. and K.J. Reddy. 1994. Removal of
Nitrates from Contaminated Ground Water Using Reduction Process in Bench
Scale Reactor. Minority Science Teacher Research Initiative. University
of Wyoming, Laramie, Wyoming. Pages 72-77.
Reddy, K.J., Z. Zhang, S.P. Gloss, and P.T. Gadlin. 1994. Catalytic Reduction of Nitrate in Ground Water: A Preliminary Evaluation. Proceedings of 1994 Universities Council on Water Resources Symposium. L.W. Mays (ed) Big Sky, Montana. (in review)
Wyoming Water Resources Center
P.O. Box 3067, University Station
Laramie, WY 82071-3067
(307) 766-2143
Fax: (307) 766-3718
 |
RB94-02 |
Research Briefs List
Water Resources Data System Library |
Water Resources Data System Homepage