Wyoming Water Resources Center

|
RESEARCH BRIEFS Wyoming Water Resources Center |

|
| Technical RB92-02 |
Investigators: K.J. Reddy, Wyoming Water Resources Center and S.E. Williams, Department of Plant, Soil, and Insect Sciences, University of Wyoming.
Purpose: Although petroleum reserves are finite and perhaps shrinking, solid fossil fuels such as coal and oil shale, are abundant and increasing in importance in Colorado, Utah, and Wyoming. Coal combustion and oil retorting of shale both produce alkaline solid wastes (e.g., fly ash and spent shale), which are potential causative agents for environmental pollution of soil, surface water, and groundwater. Revegetation of these wastes is hampered by high pH, increased solubility of some chemical constituents, and very low biological activity. The objectives of this study are to (A) develop a method to lower the pH of alkaline solid wastes by CO2 exposure under pressure in the presence of moisture, and to (B) determine the effect of CO2 pressure treatment on solubility and availability of various elements and on biological activity.
Methods:
Two fly ash samples, #1 and #2, were collected from power plants in Wyoming.
Two spent shale samples, PPP6 (Paraho Pilot Process) and Lurgi, were
collected from Western Research Institute, Laramie, WY. Objective A was
met by preliminary experiments and optimization of treatment variables.
Subsequent experiments were designed with a 2-level, 3-variable factorial
 design for optimization of treatment variables. After optimization studies,
approximately 1 kg of each sample was reacted in the vessel with 20%
moisture for 5 days at 40 psi. Samples were mixed well to ensure complete
recarbonation. After treatment, samples were transferred into plastic
bottles for subsequent experiments. To meet objective B, treated and
untreated samples were subjected to chemical studies (X-ray diffraction,
AB-DTPA extractable, and CaCO3 analysis) and biological studies. For
biological studies, treated and untreated samples were plated on several
media specific to general groups of soil microorganisms. Media used were
glucose nutrient agar (for fast growing bacteria), potato dextrose agar
(for fungi), Lingappa and Lockwood medium (for actinomycetes) and modified
mineral salt medium (for slow growing bacteria). In addition, yellow
blossom sweet clover was grown in dilutions of each material (undiluted,
1:5, 1:10, and 1:100). Plant health, nodule number, and infection by
vesicular-arbuscular mycorrhizae were recorded.
design for optimization of treatment variables. After optimization studies,
approximately 1 kg of each sample was reacted in the vessel with 20%
moisture for 5 days at 40 psi. Samples were mixed well to ensure complete
recarbonation. After treatment, samples were transferred into plastic
bottles for subsequent experiments. To meet objective B, treated and
untreated samples were subjected to chemical studies (X-ray diffraction,
AB-DTPA extractable, and CaCO3 analysis) and biological studies. For
biological studies, treated and untreated samples were plated on several
media specific to general groups of soil microorganisms. Media used were
glucose nutrient agar (for fast growing bacteria), potato dextrose agar
(for fungi), Lingappa and Lockwood medium (for actinomycetes) and modified
mineral salt medium (for slow growing bacteria). In addition, yellow
blossom sweet clover was grown in dilutions of each material (undiluted,
1:5, 1:10, and 1:100). Plant health, nodule number, and infection by
vesicular-arbuscular mycorrhizae were recorded.
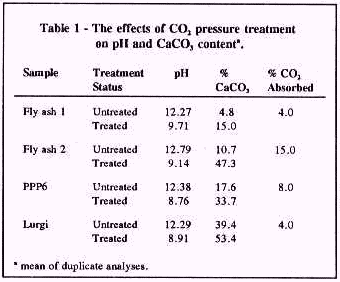
Results: Fly ash and spent shale samples were alkaline (Table 1). Combustion of coal and oil shale at high temperatures causes decomposition of carbonate minerals and production of oxides and silicate phases. Rapid reaction of oxide and silicate phases with water caused a high pH of untreated samples. Reacting samples at 40 psi with 20% moisture for 5 days lowered pH to around 9.0 as a result of calcite precipitation. The data presented in Table 1 further suggest that fly ash and spent shale samples are effective absorbents for CO2. Fly ash and spent shale samples had significant concentrations of environmentally deleterious elements before treatment (Table 2). The CO2 treatment effectively reduced these concentrations (Table 2).
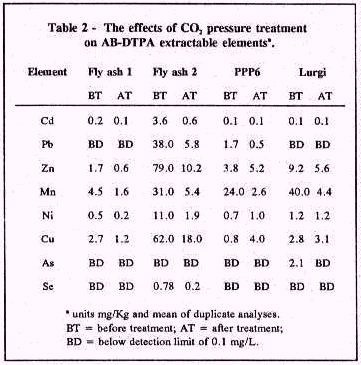 Plate counts of the microbial populations in the fly ash and spent shale were
dominated by bacteria. However, absolute numbers of bacteria strongly
suggest that these materials. Both treated and untreated, are very low in
biological activity. Very few fungi were recorded and almost no
actinomycetes were observed.
Plate counts of the microbial populations in the fly ash and spent shale were
dominated by bacteria. However, absolute numbers of bacteria strongly
suggest that these materials. Both treated and untreated, are very low in
biological activity. Very few fungi were recorded and almost no
actinomycetes were observed.
Conclusions: Reacting fly ash and spent shale samples at 40 psi for 5 days with 20% moisture lowered the pH from 12.0 to 9.0 by the precipitation of calcite. The yellow blossom sweet clover bioassay demonstrated that this plant could survive in most of the materials, treated or untreated. However, these data require further analysis. CO2 pressure treatment effectively decreases the release of various elements from alkaline solid wastes. The significant aspect of this process is the use of CO2, which is obtained either from the combustion process itself or from another source. Another potential benefit of the process is that it may help to reduce emissions of CO2 into the atmosphere. However, further research is needed to examine the long term stability of treated samples.
Publications:
Reddy, K.J., J.I. Drever, and V.R. Hasfurther. 1991. Effects of a CO2 pressure process on the solubilities of major and trace elements in oil shale solid wastes. Envir. Sci. and Tech. 25: 1466-1469.
Reddy, K.J. 1991. Immobilization of toxic elements in alkaline solid wastes. Fourth annual Meetings of Amer. Water Resourc. Assoc., Wyoming section, Laramie. p10-12.
Clark, R.A., K.J. Reddy and L. Wang. 1991. Utilization of CO2 to minimize the solubilities of Toxic elements in alkaline solid wastes. Envir. Qual. Div. Agron. Abst., Madison, Wiconsin. p38.
Reddy, K.J. and S.E. Williams. 1991. Development of a chemical and biological
method to Reclaim alkaline solid wastes. Final Rep., U.S.G.S., Section 104.
| The Wyoming Water Resources Center publishes two series of RESEARCH BRIEFS. The more technical series is designated by Technical in front of the RESEARCH BRIEF publication number. For further information on this or other research projects or for a list of WWRC publications, telephone or write: |
Wyoming Water Resources Center P.O. Box 3067, University of Wyoming Laramie, WY 82071-3067 (307) 766-2143; FAX (307) 766-3718 |
| RESEARCH BRIEFS are published by the Wyoming Water Resources Center with funds provided in part by the US Geological Survey, Department of Interior, as authorized by the Water Resources Research Act of 1984. The research on which this report is based was financed in part by the US Geological Survey, Department of Interior and Wyoming Water Resources Center. The views expressed do not necessarily represent those of the Department of Interior or the WWRC. Persons seeking admission, employment, or access to programs at the University of Wyoming shall be considered without regard to race, color, national origin, sex, age, religion, political belief, handicap, or veteran status. |
 |
Technical RB92-02 |
Research Briefs List
Water Resources Data System Library |
Water Resources Data System Homepage